doi: 10.20986/resed.2021.3841/2020
Review
Inflammatory mediators: its connection with chronic pain and associated problems. Review
Mediadores inflamatorios: su relación con el dolor crónico y problemas asociados. Revisión bibliográfica
L. Crespo-Pardo1
Y. Taboada-Iglesias1,2
1Facultad de Fisioterapia. Universidad de Vigo, Pontevedra, España
2Facultad de Ciencias de la Educación y del Deporte. Departamento de Didácticas Especiales. Universidad de Vigo, Pontevedra. Grupo de investigación en Educación, Actividad Física y Salud. (Gies10-DE3). Instituto de Investigación Sanitaria Galicia Sur (IIS Galicia Sur), SERGAS-UVIGO. Vigo, España
ABSTRACT
Introduction: Pain is a complex interaction of systems, with a process that integrates sensory, cognitive and/or emotional information that comes from real or potential threats. It has been observed a connection in the chronic pain with other somatic and psychological symptoms, such as depression, anxiety, lack of sleep, fatigue and reduction of cognitive capacity. It has been demonstrated that proinflammatory cytokines and associated symptoms influence in the probability of suffering chronic pain. The objective is to analyze the levels of the inflammatory mediators in diseases with chronic pain and with related disorders, so that we can better understand long-term pain.
Material and methods: To make this dissertation, a review of the scientific literature was carried out by a systematized search in the Pubmed, CINHAL, Medline, Scopus and Web of Science databases, in January 2020. The inclusion criteria were: to be documents written in English or Spanish and published in the last
5 years. The exclusion criteria were: review studies, and articles repeated in other databases or that addressed other topics.
Results: 13 articles were selected after applying inclusion and exclusion criteria. All publications were observational studies. Proinflammatory cytokine values were compared in patients with chronic pain, taking into account insomnia, pain tolerance, catastrophism, gender and body mass index.
Conclusion: Chronic pain is conditioned by multiple factors, so its physiopathology must be known. In this way, the measurement of the C-reactive protein and the proinflammatory cytokines could mean an advance in the evaluation and monitoring of the patient with chronic pain.
Key words: Chronic pain, inflammatory cytokines, insomnia, C-reactive protein.
RESUMEN
Introducción: El dolor es una compleja interacción de sistemas, con un proceso que integra información sensorial, cognitiva y/o emocional proveniente de amenazas reales o potenciales. En el dolor crónico se ha observado una relación con otros síntomas somáticos y psicológicos como la depresión y la ansiedad, falta de sueño, fatiga y disminución de la capacidad cognitiva. Se ha demostrado que las citoquinas proinflamatorias y los síntomas asociados influyen en la probabilidad de padecer dolor crónico. El objetivo es analizar los niveles de los mediadores inflamatorios en patologías que cursan con dolor crónico y los trastornos relacionados para así comprender mejor el dolor de larga duración.
Material y métodos: Para la realización de este trabajo se realizó una revisión de la literatura científica mediante una búsqueda sistematizada en enero de 2020 en las bases de datos Pubmed, CINHAL, Medline, Scopus y Web of Sciencie. Se aplicaron como criterios de inclusión: ser documentos publicados en los últimos 5 años y estar escritos en inglés o castellano. Como criterios de exclusión: estudios de revisión, artículos repetidos en otras bases o que trataran sobre otro tema.
Resultados: 13 artículos fueron seleccionados tras aplicar los criterios de inclusión y exclusión. Todas las publicaciones son estudios observacionales. Se compararon los valores de citoquinas proinflamatorias en pacientes con dolor crónico, teniendo en cuenta el insomnio, la tolerancia al dolor, el catastrofismo, el género y el índice de masa corporal.
Conclusión: El dolor crónico está condicionado por múltiples factores, por lo que se debe conocer la fisiopatología del mismo. De esta manera, la medición de la proteína C reactiva y de citoquinas proinflamatorias podría suponer un avance en la evaluación y seguimiento del paciente con dolor crónico.
Palabras clave: Dolor crónico, citoquinas inflamatorias, insomnio, proteína C reactiva
Correspondence: Yaiza Taboada-Iglesias
yaitaboada@uvigo.es
Received: August 19, 2020
Accepted: December 2, 2020
INTRODUCTION
The cause of musculoskeletal pain has historically been associated with an injured tissue that sends painful neuronal inputs to the central nervous system for pain perception. However, there should not always be a relationship between pain and nociception, or between pain and tissue damage (1). Currently, the IASP (International Association for the Study of Pain) says that “pain is an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage” (2).
Pain is defined as a complex interaction of homeostatic systems in response to an identified threat (3). Therefore, pain should be considered as a process that integrates sensory, cognitive and/or emotional information from actual or potential threats (4). This threat assessment process is performed by a set of cortical and subcortical structures called pain neuromatrix (5). However, we must differentiate acute pain from chronic pain (CP). The basis of acute pain is the inflammatory response, that is, a harmful stimulus or tissue damage leading to the release of chemical algogens, such as prostaglandins, bradykinin, tumor necrosis factor (TNF), neural growth factor, histamine, substance P and the calcitonin gene-related peptide. This inflammation will sensitize the nociceptor and increase the generation and transmission of stimuli, known as peripheral sensitization, decreasing the nociceptive threshold and facilitating nociceptive responses to promote tissue recovery (6). In contrast, CP is characterized by sensitization of the spinal cord, activation of the nociceptive pathways projecting into the spinal cord and midbrain, and activation of the descending pain facilitation systems and loss of descending inhibition, for the maintenance of sensitization (7).
People with emotional problems, behavioral problems, excessive alcohol use, or sleep disorders have been shown to be more likely to develop musculoskeletal pain in the medium or long term (8). In fact, CP has a devastating effect on many aspects of daily life, reducing the patient’s quality of life by negatively impacting on the physical and emotional health (9). According to a European survey, half of patients with CP feel tired all the time and 40% patients feel helpless or unable to think or function normally (10). In addition, 62% patients reported a lack of awareness and knowledge of the disease in their environment, and 47% patients believed that the rest of the people doubted the real existence of their pain (9).
A clinical entity, generalized chronic pain (GCP), is defined as generalized body pain, and often includes other somatic and psychological symptoms such as depression and anxiety, lack of sleep, fatigue, and decreased cognitive ability (11). Therefore, beliefs and emotions are capable of activating neuromatrix, provoking and even perpetuating pain without the need for nociception (12).
Sleep problems, including insomnia, are a common complaint among adults with CP (13). These alterations have been associated with higher levels of circulating inflammatory cytokines (14), which also vary in response to stress (15). In addition, various research studies have confirmed the positive correlation between catastrophism, pain intensity and perceived disability (16,17).
Similarly, increased sensitivity to experimental pain (hyperalgesia) has been found in conditions of CP. Compared with healthy subjects, patients with CP have low pain thresholds, which implies a change in perceived sensations with increased pain after a non-painful stimulus (allodynia), decreased pain tolerance and increased pain rates in response to experimental pain stimuli (18). In this sense, generalized hyperalgesia has been associated with C-reactive protein (CRP), revealing an association between CRP and experimental pain tolerance (19). In addition, after peripheral nerve injury, macrophages and Schwann cells, which gather around the injured nerve area, release cytokines and specific growth factors needed for nerve regeneration. Localized inflammatory irritation of the dorsal root ganglion (DRG) not only increases proinflammatory cytokines, but also decreases anti-inflammatory cytokines (20,21).
CRP can be synthesized in the liver and other cells of tissue such as the kidney (22), lung (23), nervous system (24), adipocytes (25) and leukocytes (26). CRP is expressed as a result of several stimuli, such as IL-6 and IL-1, being considered a classic acute phase protein. The level of CRP is acceptably correlated with the severity of inflammation, making it a reliable marker of infections, inflammation, and response to treatment (27) and therefore an inflammatory marker associated with pain.
In summary, although acute pain is traditionally related to inflammation, the aim of this study is to analyze the behavior of inflammatory mediators (IM) in diseases involving chronic pain and disorders associated with that disease, so that we can understand the long-term pain and all the mechanisms influencing it, so that we can approach it more appropriately.
MATERIAL AND METHODS
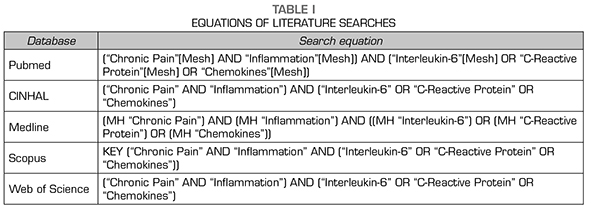
A search was performed on PUBMED, Scopus, Web of Science, Medline and CINHAL in January 2020 to conduct the present study. The same Medical Subject Headings (MeSH) terms were used in the used databases: “Chronic Pain”, “Inflammation”, “Interleukin-6”, “C-Reactive Protein” and “Chemokines” (Table 1).

To obtain valid results, the inclusion criteria were studies published in the last 5 years and written in English or Spanish. The exclusion criteria were: Review studies, articles repeated on other databases or dealing with another topic. The results and the search process are summarized in Figure 1.
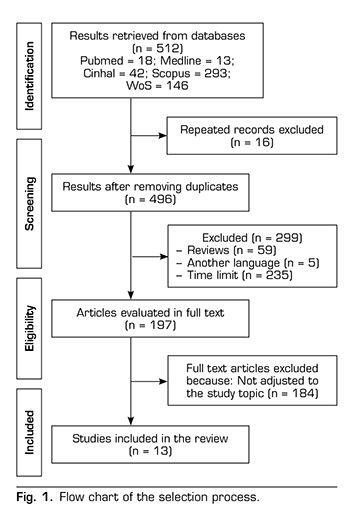
RESULTS
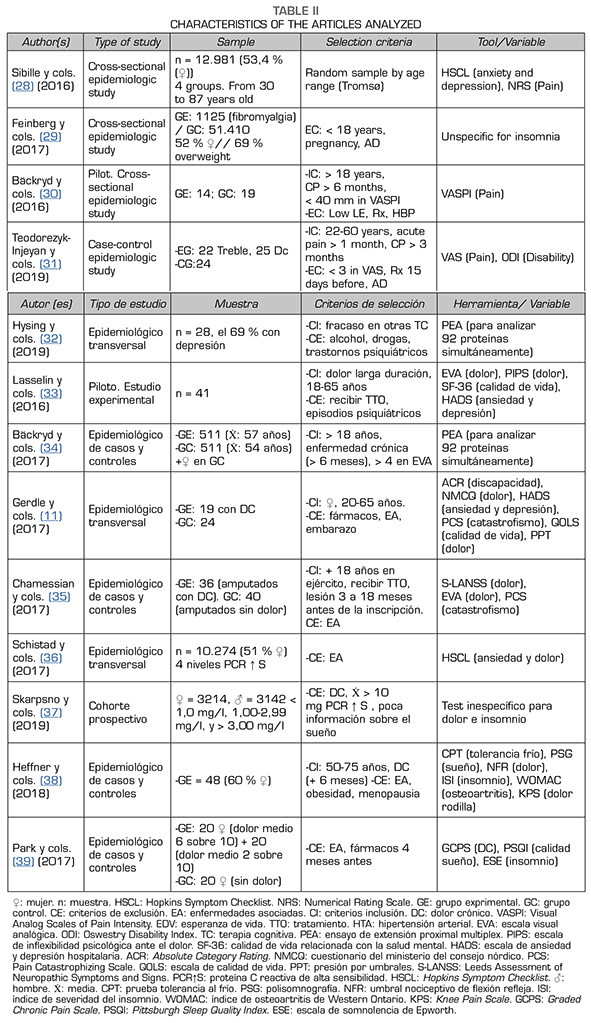
In the presentation of the results, a table has been created with the aim of facilitating the understanding of methodological characteristics. In addition, it has been sought to establish a logical order based on the different analyzes and variables to facilitate their understanding and subsequent analysis. These methodological characteristics are described in Table 2.
DISCUSSION
All the articles included in the present review are observational studies, because this is a topic that is in the early stages of research.
Thanks to the tools used, high IM levels have been significantly correlated with high levels of disability (31), psychological unhappiness (33), poor quality of life (33), high pain intensities and severity (11,35), high catastrophizing dates (11,35) and insomnia (37,38,39). Therefore, the association of chronic pain with comorbidities is present and increases the risk of suffering it.
PAIN AND IM
There is evidence showing that IL-6 contributes to the development of neuropathic pain behavior after peripheral nerve injury (40,41). Bäckryd et al. (30) found statistically significant IL-6 levels in patients with neuropathic pain. However, a cohort study with a larger sample size indicates that this cytokine shows no outstanding values one year later, where they found increases in CC and IL-8 (34), which have also been related to neuropathic pain (42).
Although IL-6 is the most studied cytokine in relation to nerve pain, it is not the most significant value shown in the study by Chamessian et al. (35), where experimental patients experienced chronic pain due to injury from trauma to the stump nerve. Significantly higher levels were in TNF, IL-8, PCR, and growth factor-related proteins. In addition, it is important that the CRP has shown high values, because, despite being considered a marker in the early stages of inflammation, it is also considered a marker that allows the evaluation and monitoring of the development of inflammatory progression (27,43).
There is abundant evidence that certain proinflammatory cytokines such as IL-1β , IL-6, and TNF are involved in the process of pathological pain (21). This group of mediators has been analyzed by Teodorezyk-Injeyan et al. (31), who found higher values in the group of patients with chronic low back pain compared to the control group. In addition, isolated TNF also has shown significant differences between patients with chronic and acute low back pain. Lasselin et al. (33) also found significant values, but in this case behavioral therapy significantly reduced serum TNF concentrations. Both studies showed an association between pain and TNF, as their values decrease after the intervention or are lower in patients with no pain. It should be noted that TNF acts in various signaling pathways through its receptors in glial cells (44) and plays important roles in both inflammatory and neuropathic hyperalgesia (21).
Hysing et al. (32) also support the benefit of behavioral therapy in cytokines in the IL-8 group. In addition, in this study, 4 cytokines increased after treatment. This increase, such as the baseline value of chemokines expressed in acute phases, may be because, in contrast with rheumatic diseases, such as osteoarthritis (OA) or ankylosing spondylitis, chronic pain is not an inflammation in itself. In addition, in this study more than half of patients have associated problems, such as depression and/or anxiety, being associated processes that can contribute to the release of pro-inflammatory cytokines in patients with chronic pain, even in diseases not considered inflammatory.
Heffner et al. have also conducted a behavioral therapy study (38) showing a significant lower level of IL-6 after the intervention. One of the inclusion criteria was that participants had to have medical evidence of knee OA and pain for more than 6 months, so in this case inflammatory cytokines are associated with a properly inflammatory but chronic disease.
It has been observed in animal studies that localized inflammation in the DRG upregulates a number of pro-inflammatory cytokines, including IL-6, and induces an abnormal sympathetic response in the absence of peripheral nerve injury (20). In inflammatory diseases, such as OA, the role of the sympathetic nervous system may increase the patient’s painful sensation due to peripheral sensitization caused by chemical stress associated with perivascular nerve growth (45).
As with neuropathic pain, IL-1β , IL-6, and TNF levels are also increased in patients with TMD from the Park et al study. (39). However, in these patients, despite having higher CRP values compared to the control group, the levels of the CRP are not as decisive or significant. TMD have been correlated with hyperalgesia in areas peripheral to injury (46), so the values of the analyzed inflammatory cytokines are understood due to their role in the process of pathological pain (21).
Finally, chronic pain has also been associated with increased and statistically significant levels of CRP in the studies of Sibille et al. (28), Feinberg et al. (29) and Skarpsno et al. (37). Given the large number of participants in the three studies and the relevance of CRP to pain mechanisms, because it is influenced by several chemokines, CRP can be considered as one of the most relevant markers and with more association to chronic pain, because CRP has been associated with generalized hyperalgesia, revealing an association between experimental pain tolerance and this protein (19).
TOLERANCE TO PAIN AND IM
Pain tolerance tests were used by Gerdle et al. (11), and the results were related to increased cytokines, including CC-type chemokines and IL-1β , which regulate and activate the pain signaling pathway, and, in particular, IL-1β increases the production of substance P in several neuronal and glial cells (47), this leads to an increase in peripheral sensitization and therefore decrease pain tolerance (6). This association leads us to analyze the study conducted by Schistad et al. (36), who show that hs-CRP levels are correlated with chronic pain patients, and were less tolerant to cold than the other groups. Considering the large sample size of this study, with 10,274 patients, and data reported by Gerdle et al. (11), an association is found between IM and patients with chronic pain, as well as with decreased pain tolerance.
INSOMNIA AND IM
Insomnia has been shown to be associated with IM due to increased IM when this clinical entity is stressed (13,14). For this reason, authors such as Skarpsno et al. (37), Heffner et al. (38) and Park et al. (39) analyzed the relationship that might be in their studies.
High levels of insomnia and high hs-CRP values were found to be associated with a higher likelihood of chronic pain (37). Park et al. (39) also related the worst sleep indicators to the higher pain and higher IM score groups. However, behavioral therapy was shown to be a technique that improves insomnia and significantly decreases IL-6 levels (38). Current evidence defines IL-6 as the most sensitive inflammatory mediator for sleep changes (48).
Some studies have shown that sleep restriction can induce an inflammatory response (48,49), which can contribute to the sensitization of the nociceptive system and thus increase susceptibility to the development of chronic pain (49).
As for the duration of sleep, the IM that shows the greatest association is the CRP. Sleep disorders is believed to have proximal effects in IL-6; but, as we discussed above, IL-6 induces CRP. Therefore, the increase in CRP could be due to a more persistent or severe sleep disturbance (48).
It is difficult to draw solid conclusions about sleep disorders by examining three articles, given the variety of differences between the methodology and inflammatory markers. But despite this, current evidence supports the extracted data, and treatment of insomnia has been found to reduce inflammation (50,51). Therefore, sleep should be a clinical entity to consider in the treatment of patients with chronic pain.
GENDER, INSOMNIA AND IM
In eight (11,28,29,34,36,39) of the thirteen studies included in this review, there are a larger number of women or only women were included. The fact that there are more women could be because the results indicate that women are more likely to have chronic pain due to factors such as menstrual cycle, temporal summation of pain, endogenous inhibitory mechanisms of pain, and gender beliefs and expectations (52). Moreover, the past individual medical history influences the painful response in women rather than men, in short, other psychosocial factors could contribute to differences in pain sensitivity between men and women. Sibille et al. (28) found that chronic pain in women was significantly higher than in men, compared with the control group.
Sex differences, predominantly female, exist in sleep quality, duration, latency, and insomnia (53). However, complaints of insomnia and daytime sleepiness are quite more frequent in women, with 58% compared with 42% in men (54). However, Heffner et al. (38) showed that their male patients had more insomnia than women, although this difference was not significant. Despite this, it appears that women may be more vulnerable to the effects of sleep disorder and show higher increases in IL-6 and CRP (55).
ANXIETY, DEPRESSION, AND IM
A stimulation of the immune system and increased secretion of immune parameters such as IL-6 and TNF are associated with a negative mood (56,57). Increased cytokine concentrations and insomnia have also been associated with the risk of depression (58,59,60).
It has been proposed that the factors generating stress initiate a cascade of biochemical events that also involves the participation of IM of the immune system, cooperating all of them in the appearance and expression of depression (61).
This review suggests that behavioral therapy reduces TNF levels, but that in only patients with low inflammatory status it may reduce unhappiness (33). It also produced improvements in inflammatory levels in patients with anxiety and depression (32). In spite of this, the sample in both studies is small, so the claims should be taken with caution.
Sibille et al. (28) show that chronic pain did not correlate depression and anxiety. Schistad et al. (36) found no association with inflammatory levels using the same assessment scale. Only Teodorezyk-Injeyan et al. found an association between anxiety and depression and IM (31), therefore, although the evidence has found an association between increased IM values and the risk of such clinical symptoms, the data are inconclusive.
It was concluded that there are significantly higher statistical values in patients with chronic pain than in the control group in some protein or inflammatory marker analyzed. Despite the variability of the mediators analyzed, CRP has been the most analyzed protein in all articles, followed by: TNF, IL-6, IL-8 and IL-1β ; ordered from highest to lowest number of occurrences in the articles. Early diagnosis would help improve treatment.
In the same way it is shown association of suffering chronic pain with increased values of the IM, which in turn decrease the tolerance to the pain. It is also related to insomnia, with a higher prevalence in women and obesity. Contrary to the previous evidence, no significant association with anxiety and depression has been found in these articles.
CONCLUSIONS
Therefore, the measurement of CRP and proinflammatory cytokines could lead to an advance in the assessment and follow-up of the patient with chronic pain. Due to the current difficulty in making these measurements in clinical practice, healthcare professionals must understand the pain and all associated mechanisms that should be considered for proper treatment and management of the pain.
FUNDING
The present study was not performed using any funding source, so there are no such conflicts.
REFERENCES