DOI: 10.20986/resed.2021.3900/2021
REVIEW
GENICULAR NERVE RADIOFREQUENCY IN OSTEOARTHRITIS-RELATED CHRONIC KNEE PAIN
RADIOFRECUENCIA DE LOS NERVIOS GENICULADOS PARA EL TRATAMIENTO DEL DOLOR CRÓNICO EN LA OSTEOARTROSIS DE RODILLA
J. C. Pérez Moreno1
D. C. Nájera Losada1
M. Herrero Trujillano2
R. Gálvez Mateos1
M. A. Sánchez García1
A. Vela de Toro1
R. López Martín1
1Unidad del Dolor. Hospital Universitario Virgen de las Nieves. Granada, España
2Unidad del Dolor. Hospital Universitario Puerta de Hierro. Majadahonda, España
ABSTRACT
Introduction: The use of genicular nerve radiofrequency procedures to treat chronic knee pain due to osteoarthritis has surged in 2011, though many questions remain regarding anatomical targets, selection criteria, and evidence for effectiveness.
Materials and methods: An electronic search was performed from January 2011 to April 2020. Databases searched included PubMed®, Embase®, Google Scholar and Web of Science (WoS). The initial search found 106 articles. Thirty-three articles were taken for this review.
Results: After analyzing five open clinical trials, one cross-sectional study, four prospective observational studies, eight neuroanatomy studies, three retrospective studies, four clinical cases, two case series, three literature reviews and three randomized, double blind, controlled trials; we found genicular nerve radiofrequency achieves a pain reduction and functional improvement with a variable duration, between three and twelve months. There is no consensus regarding the neuroanatomy of the knee joint capsule, the location of the targets, the radiofrequency parameters used and the usefulness of diagnostic blocks.
Conclusion: More clinical trials are needed to standardize the parameters used and confirm the positive results of genicular nerve radiofrequency. Although there are few cases of adverse events associated with radiofrequency of the geniculate nerves, more studies are needed to support the safety of this technique and its long-term side effects in osteoarthritis knee pain management associated that do not respond to other previous medical treatments.
Key words: Knee pain, osteoarthritis, radiofrequency, ablation, denervation, genicular nerve
RESUMEN
Introducción: El uso de radiofrecuencia de los nervios geniculados para el tratamiento del dolor crónico de la rodilla secundario a osteoartrosis inició en 2011, y desde entonces se han realizado varios estudios con diferentes metodologías. Sin embargo, continúan generándose muchas dudas con respecto a las dianas anatómicas, los criterios de selección y la evidencia de su efectividad.
Materiales y métodos: Se realizó una búsqueda electrónica desde enero de 2011 hasta abril de 2020 en las siguientes bases de datos: PubMed®, Embase®, Google Académico y Web of Science (WoS). La búsqueda inicial encontró 106 artículos, de los cuales tomamos 33 para realizar la presente revisión.
Resultados: Después de analizar cinco ensayos clínicos abiertos, un estudio de corte transversal, cuatro estudios prospectivos observacionales, ocho estudios de neuroanatomía, tres estudios retrospectivos, cuatro casos clínicos, dos series de casos, tres revisiones de la literatura y tres ensayos clínicos aleatorizados, controlados y doble ciegos; encontramos que la radiofrecuencia de los nervios geniculados disminuye el dolor asociado a la osteoartrosis de rodilla, consiguiendo una mejoría funcional con una duración variable del efecto analgésico entre tres y doce meses. A pesar del avance científico en esta área, aún no hay un consenso en cuanto a la neuroanatomía de la cápsula articular de la rodilla, la ubicación de las dianas, los parámetros empleados en radiofrecuencia y la utilidad de los bloqueos diagnósticos.
Conclusiones: Se necesitan más ensayos clínicos que estandaricen los parámetros utilizados y confirmen los resultados positivos de los estudios realizados con radiofrecuencia de los nervios geniculados. Aunque son pocos los casos de eventos adversos asociados a la radiofrecuencia de los nervios geniculados, necesitamos más estudios que avalen la seguridad de esta técnica y sus efectos secundarios a largo plazo en el tratamiento del dolor crónico de la rodilla secundario a osteoartrosis que no responde a otros tratamientos.
Palabras clave: Gonalgia, osteoartrosis, radiofrecuencia, ablación, denervación, nervios geniculados
Received: 08-02-2021
Accepted: 17-04-2021
Correspondence: John Carlos Pérez Moreno
johncarlosperezmoreno@gmail.com
INTRODUCTION
Osteoarthritis (OA) is one of the most common types of arthritis in adults, being the leading cause of musculoskeletal pain and locomotive disability worldwide (1). Knee OA (gonarthrosis) is one of the main causes of gait limitation in the population of older people in Europe; finding a prevalence of 13.83 % of knee OA in Spain in patients over 40 years of age (2).
The treatment of OA is aimed at reducing pain, increasing joint mobility, reducing physical disability, improving quality of life, limiting progression of joint damage and promoting patient education in the management of this disease (3). The combination of pharmacological and non-pharmacological modalities is required to achieve these therapeutic objectives.
Among the non-pharmacological modalities we find one of the pillars of the treatment of OA: The programs of muscle strengthening, cardiovascular training and mental strengthening exercises (such as Tai Chi or Yoga), associated with diet-based weight control programs (1).
With pharmacological treatment, we must take into account cardiovascular (CV) and gastrointestinal (GI) comorbidities and the presence of depression. Non-steroidal anti-inflammatory drugs (NSAIDs) are initially recommended via local (topical) administration, regardless of the patient's associated pathologies. If the patient has a GI comorbidity associated, COX-2 inhibitors or non-selective NSAIDs associated with a proton pump inhibitor should be used; if there is CV comorbidity, NSAIDs are not recommended. If pain is uncontrolled with NSAIDs, we can use intra-articular corticosteroids for short-term pain relief (four to six weeks) or intra-articular hyaluronic acid if a long-term analgesic effect (beyond twelve weeks) is wanted with a favorable safety profile in case new infiltrations are required. When associated depression is present, it is recommended to start using duloxetine. Paracetamol treatment is not recommended due to lack of effectiveness and the risk of hepatotoxicity. The use of oral or transdermal opioids is also not recommended due to the low benefit in this disease and the risk of drug dependence (1). If pain is not controlled with the treatment, total knee arthroplasty (TKA) should be considered (3). Although we have to consider that TKA does not guarantee total pain relief because severe pain persists in up to 15 % of patients after TKA (4).
Certain patients who have comorbidities are not considered for joint replacement, and others who do not wish to undergo surgery or are on surgical waiting lists or have persistent pain after TKA, whom should be offered the possibility of an interventional treatment to control their pain with the thermal radiofrequency (RF) of the genicular nerves (GN) (5).
Before deepening the RF of GN, let us remember the basic principles of this technique. The RF signal produces two types of fields at tissue level: An electrical field and a magnetic field. At 500 KHz a magnetic field is negligible, the electric field being the source of all the effects observed in the injury produced by RF. The electric field produces forces in ions and other electrically charged structures, generating ionic movements, electrical currents, stress of membranes and cellular substructures. The generated current produces ionic friction, heat and temperature rise, producing nerve destruction above 45 °C. All these mechanisms, and not just the increase in temperature, have the potential to produce structural changes in the architecture of the nerve when the electric field is high enough (6). It is also important to know the factors that modify the size and shape of the lesion generated by RF, within which we find: Diameter of the needle, temperature reached, duration of the thermal radiofrequency (TRF), length and proximity of the active tip of the electrode to the target tissue (7,8).
Since 2011, when Choi et al. (9) published the first clinical trial of thermal radiofrequency of genicular nerves (TRFGN) for the treatment of chronic pain in severe knee OA that was not controlled by other conservative measures, several clinical cases, neuroanatomy studies, sonoanatomy of the knee have been published, and new clinical trials are being developed. The aim of this publication is to review the neuroanatomy and available literature of genicular nerve radiofrequency for the treatment of pain in knee OA.
MATERIALS AND METHODS
We conducted an electronic search from January 2011 to April 2020 in PubMed®, Embase®, Google Sholar and Web of Science (WoS) with the following keywords: Radiofrequency, ablation, denervation and genicular nerves.
The initial search retrieved 106 articles. We limited the search to publications in English or Spanish, we excluded communications to conferences, denervation studies with neurolytic chemicals, intra-articular knee radiofrequency studies, reviews of surgical techniques related to genicular nerves, use of TRFGN in post-traumatic or post-surgical pain management (e.g. in knee arthroplasties or knee arthroscopies) and clinical cases that did not provide additional information.
RESULTS
After limiting the initial search, we obtained 33 studies to perform the present literature review: Five open clinical trials, one cross-sectional study, four prospective observational studies, eight neuroanatomy studies, three retrospective studies, four clinical cases, two case series, three literature reviews, and three randomized, controlled, and double-blind clinical trials.
Neuroanatomy of the knee
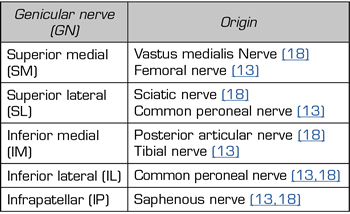
Although there is general agreement that the branches that innervate the knee joint capsule come from the femoral, sciatic, and obturator nerves, there is no consensus on the origin and number of branches innervating this area (Table I). To understand the innervation of the knee joint capsule, we divide it into two compartments: An anterior and a posterior (10). The innervation of the posterior articular capsule originates from the tibial nerve and the posterior division of the obturator nerve (11,12). The innervation of the anterior articular capsule (Figure 1) is divided into 4 quadrants (13,14): The lateral superior quadrant is innervated by the vastus lateralis nerves (VLN), vastus intermedius nerve (VIN, superior lateral genicular nerve (SLGN), and common peroneal nerve (CPN). The inferolateral quadrant receives innervation of the inferior lateral genicular (ILGN) and recurrent peroneal (RPN) nerves. The medial superior quadrant is innerved by the nerves of the vastus medialis (VMN), VIN, and superior medial genicular nerve (SMGN). The medial inferior quadrant receives innervation of the inferior medial genicular nerve (IMGN) and in some cases of the infrapatellar branch of the saphenous nerve (IPBSN).
Different studies that use TRF or pain control associated with knee OA do not address all of the described branches (13,14). The nerves that are blocked and then TRF is performed are the SMGN, SLGN and IMGN because they distally have constant contact points at the femur and tibia levels (10,14). ILGN is not addressed because of its proximity to the peroneal nerve (15).
Table I. Innervation of the anterior knee joint capsule

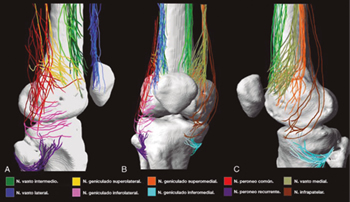
Fig. 1. Innervation of the anterior knee joint capsule. A. Lateral view. B. Anterior view. C. Medial view. Image taken from Tran et al. (13). Reproduced with the permission of Philip Peng Educational Series.
Fluoroscopy-guided genicular nerve radiofrequency
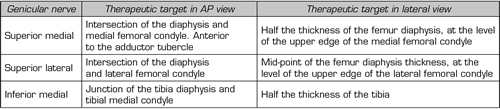
To perform a fluoroscopic-guided TRFGN, we need an anteroposterior (AP) and lateral view of the knee (Figure 2). First we place the patient in supine position with a pillow under the popliteal fossa (to make the patient more comfortable) (16). After performing asepsis and antisepsis of the knee, we proceed to locate the GN (Table II). SLGN is advancing the TFR needle toward the confluence of the lateral femoral diaphysis with the lateral femoral condyle in an AP view and at a mid-point of the femur in a lateral view. The SMGN is located by advancing the needle toward the confluence of the medial femoral diaphysis with the medial femoral condyle in an AP view and at a mid-point of the femur in a lateral view. Finally, the IMGN is located by advancing the needle toward the confluence of the medial tibial diaphysis with the tibial epicondyle in an AP view and at a midpoint of the tibia in a lateral view (16,17).
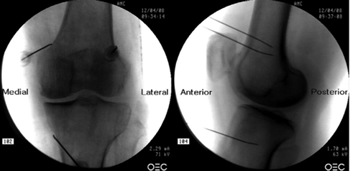
Fig. 2. Anteroposterior (AP) and lateral projection where therapeutic targets are observed using fluoroscopy. Image taken from Choi et al. (9). Reproduced with the permission of Jin Woo Shin.
Table II. Location of genicular nerves guided by fluoroscopy
Good AP and lateral view is very important. In AP projection, the tibiofemoral joint should have a similar width on both sides of the knee with the interspace open (9). In lateral projection, there must be a correct overlap of both femoral condyles to perform a satisfactory blockage of the SMGN and SLGN (18).
Most authors take the references described above to locate these nerves by fluoroscopy, except Fonkoué et al. (18), who find that the therapeutic targets of SMGN and SLGN (in a lateral view) are at the junction of the upper edge of their respective femoral condyles with the posterior cortex of the femur diaphysis, and not in half the thickness of the femur, as described classically (9,10,13).
Because the success of the TRF of the genicular nerves depends on the correct location of the RF needle tip (as close as possible to the nerve), Know et al. (17) demonstrated with magnetic resonance imaging that the points used classically in fluoroscopy for ablation of the three genicular nerves (SMGN, SLGN and IMGN) are correct. They observed that GN passes through the intersection formed by the diaphyseal and metaphysis line of the distal femur or proximal tibia (SLGN 92 %, SMGN 88 % and IMGN 100 %).
After locating the TRF needle (22G of 100 mm with 10 mm active tip) at the desired points, we proceed to confirm the position of the nerve using a 50 Hz sensitive stimulus.
The threshold of sensory stimulation at which the patient perceives paresthesia or pain should be less than 0.6 V. Then we confirm the absence of fasciculations in the lower extremity using motor stimulation at 2 Hz with 2.0 V. Finally, 2 ml lidocaine is administered at 2 % or mepivacaine 2 % in each nerve, and TRF is initiated at 80 °C for 90 seconds (9,14).
Although most studies reference a sensitivity threshold of less than 0.6 V, some authors such as Iannaccone et al. (15) used a lower sensory stimulation threshold (0.15 V) to optimize the position of the needle with good results until six months after RF. Unfortunately, we did not find studies comparing different sensory stimulus thresholds and their effect on the duration of analgesia provided by RF.
Ultrasound-guided genicular nerve radiofrequency
It is not yet clear which imaging method is superior as a guide to perform TRFGN, but there are authors, such as Kim et al. (16) that conclude that ultrasound may be the tool of choice. In their study, they found no differences in the efficacy of genicular nerve block (GNB) when performed in an ultrasound-guided or fluoroscopic-guided manner. These authors localized the genicular nerves in an ultrasound-guided manner and then performed images with fluoroscopy and contrast, showing that targets for GNB were located similarly regardless of the imaging method used. Pain relief, functional improvement using the Western Ontario and McMaster’s Universities Osteoarthritis Index (WOMAC) scale, and safety were similar in both groups (16).
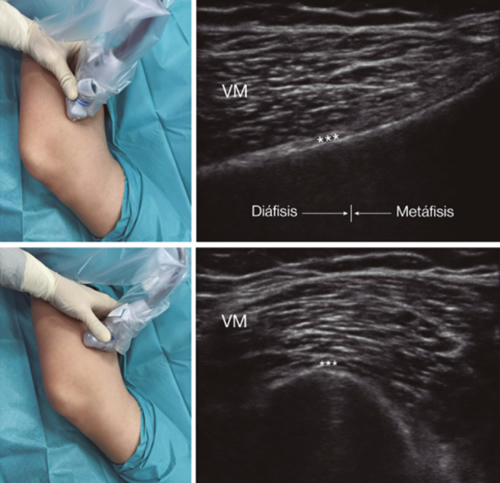
Fig. 3. Sonoanatomy and technique for performing superior medial genicular nerve block (SMGN). The transducer is located on the distal long axis of the femur and once the position of the SMGN (asterisks) is known, the probe is rotated 90 degrees to obtain a view of the femur short-axis (don't forget to keep the same depth that we find the SMGN on the long axis). VM (vastus medialis).
In order to achieve a correct location of anatomical landmarks by ultrasound we must place the patient in supine position with the knee flexed and a pillow under the popliteal fossa (14,16,19).
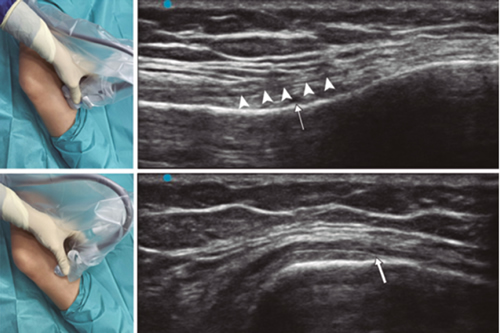
Fig. 4. Sonoanatomy and technique for performing inferior medial genicular nerve block (IMGN). The transducer is located on the proximal long axis of the tibia and we identify the vasculonervious package of the IMGN (arrow) just below medial collateral ligament (arrowheads). Then the probe is rotated 90 degrees to obtain a short-axis view of the tibia (don't forget to keep the same depth as the IMGN found on the long axis).
After performing knee asepsis/antisepsis, surgical field placement, and the sterile sheath of the high frequency linear transducer, we proceed to locate the SMGN (Figure 3). Placing the probe in a coronal plane on the inner face of the knee, we slide it cranially to visualize the junction of the metaphysis to the femoral diaphysis and the superior medial genicular artery/nerve (ASMGN), usually located near the periostium of the femur (if this neurovascular structure is not found, the junction between metaphysis and femoral diaphysis is taken as a reference). The mid-point of the transducer corresponding to ASMGN is then marked on the skin and the transducer is rotated to be placed in the transverse or axial plane to view ASMGN on the short axis (if this structure is not visible, confirm that we are at 50 % depth of the femur). In this cross-section the needle of the TRF is advanced in plane from anterior to posterior toward the ASMGN or to a depth of 50 % of the thickness of the femur. Finally, the transducer is rotated 90° again, leaving it in a coronal plane to check that the needle tip is near the ASMGN or the junction of the metaphysis and femoral diaphysis (14,16,19).
To locate the IMGN (Figure 4), we placed the transducer in a coronal plane on the inner face of the knee, sliding it caudally to identify the diaphysis junction with the tibial metaphysis and the inferomedial genicular artery/nerve (AIMGN). And we repeat the same steps we used for SMGN. If the AIMGN is not found, the reference to be taken shall be the depth of 50 % of the thickness of the tibia (14,16,19).
To locate the SLGN, the patient must be supine with the lower limb in internal rotation, obtaining good exposure to the lateral side of the thigh. We place the linear transducer in a coronal plane on the lateral side of the knee, slide it in a cranial direction to visualize the junction of the metaphysis with the femoral diaphysis and the superior lateral genicular artery/nerve (ASLGN), and repeat the same steps that we use for SMGN (14,16,19).
Then we found that the threshold of sensory stimulation at 50 Hz that triggers a paresthesia or pain is less than 0.6 V, and to avoid motor nerve damage there should be no fasciculations of the lower limb with a motor stimulus at 2 Hz with 2.0 V. If everything is correct, we administered 2 ml of lidocaine 2 % or mepivacaine 2 % in each nerve and performed the TRF at 80 °C for 90 seconds (9,14).
In our review we find other anatomical landmarks that can help us locate the genicular nerves. Yasar et al. (20) identified that the SMGN is located one centimeter before the adductor tubercle, the IMGN is located at the midpoint between the peak of the medial tibial epicondyle and the beginning of the insertion of the medial collateral ligament fibers over the tibia.
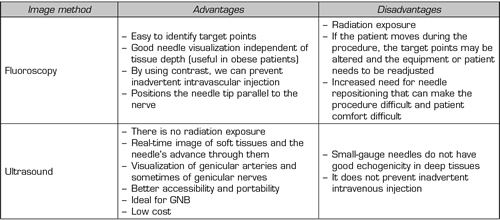
Given the advantages/disadvantages of each imaging method (Table III) and the particularities of each patient, we should choose the best tool when performing block or TRF of the genicular nerves.
Blocking of genicular nerves prior to thermal radiofrequency
Table III. Advantages of disadvantages of fluoroscopy versus ultrasound. GNB (Genicular Nerve Block)
Although most studies of TRF of genicular nerves have previously had a positive diagnostic block (described as an improvement above 50 % of baseline pain) using different doses of local anesthetic: 2 ml lidocaine 2 % (9,16,21), 1 ml bupivacaine 0.5 % (15), 1 ml lidocaine 2 % (22); it is considered a possible false positives (22), considering that 2 ml is a high volume to anesthetize the nerves and may compromise the selectivity of the blockade (14). Even some authors consider that 0.5 ml can also lead to false positives (23), others consider that blocks prior to TRF do not improve the success rate after ablation (22).
Furthermore, the role of corticosteroids associated with local anesthetics in GNB in the treatment of chronic OA pain remains controversial. Kim et al. (21) they conducted a comparative study by injecting 2 ml of a mixture of 6 ml 2 % lidocaine with 20 mg triamcinolone vs. 2 ml of lidocaine 2 % in each nerve; finding that the analgesic effect in the triamcinolone group with lidocaine was maintained for two more weeks compared with lidocaine use. These authors consider that the side effects of corticosteroids (alopecia, skin atrophy, cortisol suppression, glucose intolerance, and loss of bone density) do not compensate for the short period of additional analgesia patients receive.
In our review we found a study (24) that documented that corticosteroids are as effective as the radiofrequency of genicular nerves at 6 months of follow-up, but we have to emphasize that the dose they used in this study was three times higher than that used by Kim et al. (21). We could classify this result as a systemic analgesic effect rather than a local effect.
Although the diagnostic blocks have generated controversy, there is not enough evidence to disadvise their use at the moment. In fact, it is important to perform them to rule out pain referred to this joint.
Effectiveness of thermal radiofrequency of genicular nerves
The first study that reported improved pain in patients after TRF of the genicular nerve (SMGN, SLGN, and IMGN) was performed by Choi et al. (9). They observed a reduction in pain higher than 50 % in the first, fourth and twelfth weeks (59, 65 and 59 %, respectively) (Table IV).
Table IV. Efficacy of thermal radiofrequency of genicular nerves
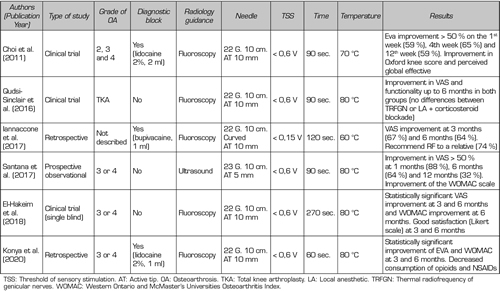
Other studies were then developed finding that pain reduction could be maintained up to one year. Iannaccone et al. (15) found not only an improvement in pain at three months in 67 % of patients, but a maintenance of this relief in 95 % of patients up to six months. Santana et al. (14) found a monthly improvement in pain in 88 % patients and at six months in 64 % of the patients studied. However, this benefit decreased progressively after six months, finding that 32 % of patients had an improvement in pain after a year, without reaching the level of pain they had prior to TRF.
Although the decrease in the pain scale is one of the most important parameters for assessing the efficacy of TRFGN, we should not forget to quantify other values that also inform us of the effectiveness of ablation of genicular nerves such as: improved functional capacity, good patient satisfaction, and decreased analgesic use. El-Hakeim et al. (25) compared TRFGN with conventional oral analgesic treatment, finding not only a statistically significant improvement in pain relief, but also an improvement in quality of life using the WOMAC scale and good patient satisfaction up to six months of follow-up. However, this study is limited by not having a pre-TRFGN prognostic block and not being a double-blind study. Konya et al. (26) found an improvement in quality of life in 79 % of patients, but also a decrease in VAS to 2 points at six months associated with a decrease in NSAID use in 40 %. achieving the abandonment of these by 50 %.
Another population that can benefit from TRFGN are patients who have pain after TKA. Protzman et al. (27) first described pain relief and improved quality of life after using ultrasound-guided and fluoroscopic-tested TRFGN in a patient with persistent knee pain after TKA. Qudsi-Sinclair et al. (24) confirmed these findings in a clinical trial conducted in 28 patients with knee pain refractory to medical treatment after TKA; the highest improvement was achieved at three months, subsequently with a progressive decrease in analgesic effect without losing it completely until one year of follow-up.
It is also important to know the predictors of success or failure of TRFGN. Factors that favor a successful technique include ostearthrosis of the medial compartment with concordant pain, previous prognostic blocks, large and/or multiple lesions. Among the factors predicting a failure of the technique are: Disease with an important burden that generates a large disability, previous surgery, use of opioids, symptoms of diffuse pain (fibromalgic) and history of psychiatric disease (28).
The studies mentioned show that TRFGN is an effective technique (in most patients up to 6 months) by decreasing VAS, increasing functionality, decreasing the dose of analgesics, and improving the quality of life of patients with knee OA.
Complications and side effects of TRF of genicular nerves
In our review, we found no patient who presented persistent weakness or neuralgia after TRFGN (15,25,26). We found published a case of a third degree burn on the area where the IMGN was located (29) and another case of septic arthritis of the knee after performing this interventional technique (30) (Table V).
Table V. Complications and side effects of TRF of genicular nerves
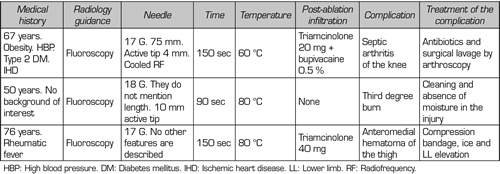
In our review, we found no patient who presented persistent weakness or neuralgia after TRFGN (15,25,26). We found published a case of a third degree burn on the area where the IMGN was located (29) and another case of septic arthritis of the knee after performing this interventional technique (30) (Table V).
Kim (31) reviewed the vascular complications of the genicular arteries described in the surgical literature. The most frequent complication was the formation of pseudoaneurysms (mainly after open synovectomies, menisectomies, arthroscopies, and total knee arthroplasties), other complications found were arteriovenous fistulas, hemarthrosis, and osteonecrosis of the patella. Fortunately, these surgical complications are not described after TRFGN, but it does not mean that there is no risk of vascular injury. In fact, a clinical case of a hematoma that was treated conservatively after an TRFGN was described (32).
In general, we can say that TRFGN is safe and the risk of a major associated complication is very low.
DISCUSSION
OA is a public health problem with a major global economic impact that may worsen over the next few years due to increased life expectancy and obesity in our population. To help counteract the impact of this disease, we must know the indications of minimally invasive techniques such as TRFGN; the candidates for this interventional technique are: Patients with grade 3-4 OA of the Kellgren-Lawrence classification with moderate to severe pain and failure of conservative treatment, patients with persistent pain after TKA and patients not candidates for surgery due to significant comorbidity or rejection of surgery (33).
Evidence suggests that TRFGN is effective and safe. TRFGN has been shown to reduce pain associated with knee OA until six months,when a progressive decrease in the effect of TRF begins to be observed (14). In our review, we only found three clinical cases reporting complications associated with this technique, so we might think it is a safe technique following the general recommendations of pain intervention.
Both analgesia and muscle relaxation obtained with ablation of genicular nerves can contribute to an improvement of the mechanical functionality of the joint allowing effective strengthening, thanks to the absence of pain during rehabilitation (14). This achievement is very important, as rehabilitation is one of the pillars of management of patients with OA.
One of the main bases of regional anesthesia and chronic pain interventionism is to have a good knowledge of the anatomy to correctly interpret the images that help us block the desired nerve structures.
Although there is no consensus regarding the origin and number of branches that instill the knee capsule, there is agreement on the distal location of the genicular nerves (mainly SMGN, SLGN and IMGN) being in close contact with the periosteum of the tibia and femur (9,10); allowing these zones to be used as therapeutic targets in our clinical practice. However, there are anatomy studies such as that of Fonkoué et al. (18) who do not agree with the classical anatomical landmarks for the TRF of the SLGN and SMGN; concluding that there is a need to review the classical references to have more precision in the ablation by TRF of these nerves. Some authors consider that Fonkoué et al. should not reach such conclusions because the performed study is not comparative (34).
In favor of classical targets we have studies such as that of Know et al. (17) showing with magnetic resonance imaging that the points used classically in fluoroscopy for ablation of the three genicular nerves (SMGN, SLGN and IMGN) are correct. In addition, we should not forget that most clinical studies have been performed with the classic anatomical landmarks, subsequently corroborated by important studies of anatomy of the knee joint capsule (10,13). The results of TRF from all these studies have been favorable: Most cases found pain improvement up to six months and in some cases, this benefit remained until one year of follow-up.
Although fluoroscopy is the most widely used radiological guide in the literature, we find that ultrasound is increasingly taking weight and several authors propose the use of ultrasound as a method of choice for GNB (16,19). The authors conclude that the use of ultrasound is ideal because there is high variability in the genicular nerve path as shown by anatomy studies (12) and the risk of radiation and injury to the genicular arteries is eliminated. (19,31) Another reason to use ultrasound is patients with persistent knee pain after an TKA because the path of the genicular nerves may change after surgery, being unpredictable its location after reinnervation (31).
In our review we found that corticosteroids associated with local anesthetics in the GNB for the treatment of chronic pain are effective, but their effect is for a short period of time (21). We should perform a risk/benefit analysis before administering corticosteroids in the GNB, bearing in mind that the TRF maintains a longer analgesia period. It may be preferable to perform a TRF with a prior positive GNB with local anesthetic as the only agent. However, the diagnostic GNB is beginning to be questioned, there are approaches to the need to redefine the selection criteria before the TRFGN (28), taking into account the number of false positives this test has (23) and that for some authors a positive block is not a predictor of the success of TRF (22).
The size of the lesion generated by TRFGN depends not only on the distance we are at from the nerve, but also on the duration of the TRF, the set temperature and the length of the active tip. No standardization of parameter is found in studies using TRFGN, for example, the length of the active tip varies between 5 mm and 10 mm, the temperature between 60 ºC and 80 °C and the duration of the procedure varies between 90 seconds and 270 seconds. This variability can lead to changes in clinical outcomes and long-term effectiveness of TRF (26). Future clinical trials should investigate which are the ideal parameters.
It is worth noting that the clinical improvement is greater in studies that have been performed with cooled radiofrequency (CRF) (22,35), probably because of the larger size of the spherical lesion generated by the CRF needle that could involve a larger number of sensitive branches (Figure 5). Indeed, Tran et al. (13) proposed to perform techniques with bipolar TRF based on their anatomy studies, where not only an ablation of the sensitive branches of the SMGN and SLGN is performed, but also of the medial and lateral branches of the VIN. In our review we found only one study comparing monopolar and bipolar TRF, without showing differences in the duration of pain relief (36).
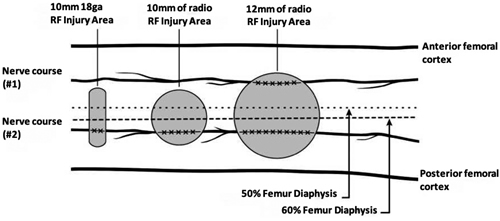
Fig. 5. The three most commonly used sizes of radiofrequency (RF) lesions (thermal RF. Bipolar RF. Cooled RF). Image obtanied from Franco et al. (10). Reproduced with the authorization of Carlo D. Franco.
We found studies using pulsed radiofrequency (PRF) at 42 °C for 5 minutes in each genicular nerve with good results, decreasing the value of the WOMAC scale and VAS, without complications or side effects (37,38). This type of RF has the advantage of not producing a nerve injury that may lead to neuropathic arthropathy or Charcot joint and secondary neuropathic pain; it is recommended in the population that does not have a very advanced knee OA. However, for some authors the use of PRF in this disease lacks of scientific basis because the type of pain associated with knee OA is not neuropathic, being less effective than the use of TRF, and the benefit shown from PRF may be a placebo effect (28). We believe that it is important to conduct clinical trials comparing different techniques and to draw conclusions based on strong scientific evidence to establish the role of genicular nerve PRF.
Finally, some authors perform RFT on other nerves such as the suprapatellar (19) and the infrapatellar (39) that are described in the anatomy studies (12,18), being interesting to include them in future clinical trials to assess whether there is an improvement in pain and a larger safety with the ablation of these nerves.
CONCLUSIONS
Further clinical trials are needed to confirm the positive results of radiofrequency studies on genicular nerves. Further studies are also needed to standardize the parameters and selection criteria used in the radiofrequency of these nerves to obtain more homogeneous samples in future studies.
Although there are few cases of adverse events associated with the radiofrequency of genicular nerves, we need more studies supporting the safety of this technique and its long-term side effects in the treatment of pain in patients with knee OA who do not respond to other previous medical treatments, or they even continue with disabling pain after total knee arthroplasty.
CONFLICT OF INTEREST
Los autores declaran no tener ningún conflicto de intereses.
REFERENCES