DOI: 10.20986/resed.2021.3882/2021
REVIEW
DORSAL ROOT GANLION PULSED RADIOFREQUENCY FOR LUMBOSACRAL RADICULAR PAIN: A NARRATIVE REVIEW
RADIOFRECUENCIA PULSADA DEL GANGLIO DE LA RAÍZ DORSAL PARA EL DOLOR RADICULAR LUMBOSACRO: UNA REVISIÓN NARRATIVA
M. Surbano and P. Castromán
Departamento y Cátedra de Anestesiología. Hospital de Clínicas. Facultad de Medicina. Montevideo, Uruguay
ABSTRACT
Objective: Pulsed radiofrequency can be a non-destructive option compared with the thermical lesion produced by continuous radiofrequency. The application of PRF of the dorsal root ganglion is a therapeutic tool in patients with Lumbosacral Radicular Syndrome refractory to epidural steroids injections. The mechanisms of action are not clear yet and a neuromodulation process is proposed. The evidence of the efficacy of this intervention is of low quality, due to several factors. Our objective is to present a review of the evidence of the efficacy and safety profile of the DRG RFP. The clinical aspects related to the technique and its possible mechanisms of action are also reviewed.
Material and methods: A bibliographic review was performed in MEDLINE (Pubmed), Google Scholar, Scopus, CINAHL, Embase, Cochrane and Fisterra (clinical guidelines) of articles in English and Spanish, during the available years with the terms: “Dorsal Root Ganglion pulsed radiofrequency” and “lumbar” or “lumbosacral radicular pain” in English and Spanish. Bibliographic review was carried out by the 2 authors independently.
Results: We present the analysis of eight prospective, randomized studies and nine cohort studies, with a before and after type of design, in which the study of efficacy and safety was proposed as the main objective.
Conclusions: This review suggested that PRF of the DRG can be a good therapeutic option in patients with refractory LRS. Larger, blinded, prospective and randomized controlled trials are needed to support this statement.
Key words: Pulsed radiofrequency, dorsal root ganglion, lumbosacral radicular pain.
RESUMEN
Objetivo: La radiofrecuencia pulsada constituye la variante no destructiva de la termolesión por radiofrecuencia. Una opción terapéutica en pacientes con síndrome radicular lumbosacro refractario a esteroides epidurales es la aplicación de RFP en el ganglio de la raíz dorsal. Si bien el mecanismo íntimo de acción de la misma no está del todo esclarecido, se plantea como una técnica de neuromodulación. La evidencia de la eficacia de esta intervención es débil por diversos factores: insuficientes estudios clínicos randomizados, el bajo tamaño muestral utilizado en los mismos, las controversias sobre algunos aspectos técnicos en la aplicación de la RFP, la selección incorrecta de pacientes, la presencia o no de dolor neuropático, etc. Nuestro objetivo es presentar una revisión de la evidencia de la eficacia y el perfil de seguridad de la RFP del GRD en pacientes con SRLS refractario. Los aspectos clínicos relacionados con la técnica y sus posibles mecanismos de acción son también reseñados.
Material y métodos: Realizamos una búsqueda bibliográfica en MEDLINE (Pubmed), Google Scholar, Scopus, CINAHL, Embase, Cochrane y Fisterra (guías clínicas) en inglés y español para todos los años disponibles con los términos “Dorsal Root Ganglion pulsed radiofrequency” y “lumbar” o “lumbosacral radicular pain” en idioma inglés y español. La evaluación fue realizada por los dos autores de manera independiente.
Resultados: Presentamos el análisis de ocho estudios prospectivos randomizados y nueve de cohorte única de tipo antes y después, de pacientes con dolor radicular lumbosacro refractario cuyos objetivos fueron la evaluación de la eficacia y seguridad de la técnica.
Conclusiones: La RFP del GRD podría ser una opción terapéutica útil en el SRLS refractario, pudiendo considerarse sus resultados como preliminares, y deberán ser confirmados por estudios prospectivos randomizados con criterios de selección homogéneos y mayor número de pacientes.
Palabras clave: Radiofrecuencia pulsada, ganglio de la raíz dorsal, dolor radicular lumbosacro.
Received: 02-01-2021
Accepted: 18-09-2021
Correspondence: Pablo Castromán
pablocastro227@gmail.com
INTRODUCTION
Menno Sluitjter introduced pulsed radio frequency (PRF) in the clinical setting, a non-destructive variant of thermal injury by radio frequency or continuous radio frequency. The first PRF on a lumbar dorsal root ganglion (DRG) was performed in February 1996, and has since been used in different neuropathic and non-neuropathic painful syndromes (1-3).
The PRF is characterized by the application of voltage in short bursts of high intensity followed by pauses of electrical silence, allowing heat dissipation. This exposes the surrounding tissue to intense electrical fields while maintaining the temperature below 42 degrees, without causing the tissue injury generated by continuous radio frequency. This allows its use on structures such as DRG, in which an injury is not admissible due to the possible neurological sequelae (4).
Lumbosacral radicular syndrome (LSRS) can occur secondary to various anatomical alterations in the lumbar spine, such as spinal stenosis, disc protrusion, disc herniation, facet osteoarthritis, or post-surgical fibrosis. It has a significant negative impact on patients’ quality of life, as do other neuropathic pain syndromes (5). Its incidence in the general population ranges from 10 % to 25 % (5). Within the interventional procedures aimed at treating pain from its pathophysiological basis are the injection of epidural steroids, with a high level of evidence in pain relief, but in the short term (6). There is a percentage of patients who are resistant to this treatment, or in whom the benefits are very short of 30 % approximately, according to some authors (6). One of the reasons for this phenomenon is the existence of a neuropathic component of pain, with little inflammatory contribution at the level of the affected nerve roots. It is suggested that DRG is intimately linked to the pathophysiological processes contributing to the origin of neuropathic pain. PRF of DRG appears to be an attractive therapeutic option in this group of patients (7).
METHODS
The objective of the present study is to review the evidence available to date on the efficacy and safety of PRF of the DRG applied to patients with lumbosacral radicular syndrome. The clinical aspects related to the technique and its possible mechanisms of action are also outlined.
In September 2020 we conducted a literature search in MEDLINE (Pubmed), Google Scholar, Scopus, CINAHL, Embase, Cochrane and Fisterra (clinical guidelines) with the terms “dorsal root ganglion pulsed radiofrequency” and “lumbar” or “lumbosacral radicular pain” and their corresponding Spanish terminology, “radiofrecuencia pulsada del ganglio de la raíz dorsal” y “dolor radicular lumbar” o “lumbosacro”. The search was limited to English and Spanish languages for all available years. The two authors independently assessed the abstracts and titles by relevance and obtained full-text versions of those articles considered relevant based on the stated objective. For the analysis of evidence on the efficacy and safety of the technique, articles in which these aspects were not within the main objective of the study (e.g., studies on mechanisms of action of the treatment) were excluded from such analysis.
Mechanisms of action
While the intimate mechanism of action in PRF is still unknown, it is stated that the application of heat does not produce neural injury, but rather the generation of a powerful electromagnetic field around the tip of the electrode, leading to morphological, biochemical and functional change in the exposed neural structure, thus being considered a neuromodulation technique (8,9).
During PRF application, current is delivered to the tissue in the form of short waves or high-voltage pulses generating heat, followed by electrical pauses or silences allowing the dissipation of generated heat, preventing the electrode tip and tissue from exceeding 42 degrees Celsius. The electric fields generated by the PRF are much more intense than in the continuous variant, but this intensity decreases rapidly ahead of the electrode tip. While high-intensity electrical fields around the tip will disperse rapidly, those generated around the nerve sheath are potent and involve a significant mass of tissue. Perhaps these fields are responsible for some degree of tissue injury described in in vitro studies and experimental animals, with minimal changes in the neural structure when compared to those produced by continuous radiofrequency.
This ability to reduce or prevent neuronal damage is at the core of the usefulness of the technique in patients with neuropathic pain (10).
Based on experimental studies, we can say that PRF produces an alternation of high and low intensity electromagnetic fields, capable of generating changes in transmembrane potential, functional involvement of ionic channels, alteration of resting potentials and generation of action potentials and reduction of ectopic discharges in neurons (11). Higuchi et al. showed that the application of PRF of the DRG in mice increases the expression of the c-Fos gene at this level, a finding similar to that found by Van Zundert et al. (12,13). The clinical relationship between c-Fos expression and pain relief has not yet been determined, but it is an indicator that this technique acts on nociceptive transmission. Other suggested mechanisms of PRF action in neuropathic and radicular pain models include interference with the release of pro-inflammatory substances at the level of disc-root conflict, attenuation of the central sensitization mechanism at the level of the dorsal horn and enhancement of the downward mechanisms of analgesia through the release of noradrenaline and serotonin (14-18).
In addition to these functional changes, in vivo experimental studies show that the application of PRF on neural tissue produces structural changes, although much smaller than those found with the application of radiofrequency at 67 degrees Celsius. Using electronic microscopy, PRF results in increased size of the endoplasmic reticulum cisternae and cytoplasmic vacuoles, while in the second case, mitochondrial degeneration and loss of nuclear membrane integrity are observed (19,20).
It is stated that DRG plays a fundamental role in the pathophysiology of radicular pain. Common causes of radicular pain, such as disc degeneration or disc herniation, lead to the release of pro-inflammatory substances, such as tumor necrosis factor α (TNF-α). These induce the production of neurotrophins that are transported from the site of the injury to the DRG. These neurotrophins would be responsible for an increase in the excitability of the neurons of the dorsal horn and for the generation of ectopic discharges in the neuronal bodies located in the DRG (11). Ectopic discharges activate microglia, which further amplifies the production of neurotrophins and the processes determining central sensitization, a pathophysiological characteristic of chronic neuropathic pain (11).
With regard to the mechanism of action of PRF of the DRG, Moore recently published a randomized study conducted in 10 patients with radicular pain, 5 of whom were treated with PRF of the DRG and the remaining 5 patients received simulation of treatment as a sham group. Patients in the PRF group had a significant decrease in pain measured using the Numeric Rating Scale (NRS) at 3 months after treatment. In cerebrospinal fluid samples from the radiofrequency-treated group, the author found a decrease in TNF-α and the number of lymphocytes CD3, suggesting a modulation of the immune system by PRF treatment (21).
There are still many questions to be answered about the different aspects of basic knowledge of this technique, which can have a significant impact on the results obtained from its clinical application. As an example, aspects such as treatment location, timing, and optimal PRF parameters remain to be determined. As for the first point, it remains to be established whether the application of PRF on a single DRG is sufficient to achieve a satisfactory beneficial result or whether, on the other side, given the divergent distribution of nociceptive afferents along several adjacent metameres, PRF needs to be applied to several dorsal root ganglia of neighboring segments. With regard to timing, it would seem clear that the modulating effects described to date are temporary, remaining to establish until now the value of repeating the procedures, taking into account that the pathophysiological alterations of chronic radicular pain are maintained over time. Finally, it is interesting to explore whether modifying PRF parameters (such as voltage, frequency, and duration of treatment), selected so far empirically, can have an impact on better results. While it would seem reasonable to maintain the electrode tip at 42 degrees Celsius, as it is the basis for safety in the use of the technique, exposure time and voltage could be the parameters to be modified. Exposure time is the most frequently modified factor (2,3). Most studies use one or more 120-second cycles, as this is considered safe, in terms of tissue injury. Thus, there are studies using 180, 240, 360 and 480 seconds of total duration (2,3). A randomized, double-blind clinical study of patients with unilateral radicular lumbar pain is currently ongoing in our department, comparing the analgesic efficacy of PRF for 240 and 480 seconds, administered in two pulses.
Other authors, such as Vigneri, suggest to increase the voltage between 65 and 80 volts to increase the efficiency of the technique while preserving the safety profile of the PRF (22,23).
RESULTS
Technical aspects
Any procedure to be performed requires first obtaining informed consent from the patient. Serious infectious complications, such as epidural abscesses or meningitis, are extremely rare in interventional treatments for chronic pain. Therefore, we have little evidence-based medicine for making recommendations for antisepsis. The measures that are indisputable are: Surgical hand washing with chlorhexidine soap, the use of sterile gloves and surgical face mask, the use of sterile fields and the antisepsis with alcoholic chlorhexidine (24). The administration of preventive antibiotics has no evidence, even in immunocompromised patients, such as diabetics and obese (24).
In spite of the increase in the use of ultrasound in interventional procedures for pain management, RF of DRG is recommended to be performed under fluoroscopy view as a first option (25).
DRG is a thickening of the dorsal root of the spinal nerve and is located below the pedicle on the roof of the neuroforamen. At the high lumbar level, it is more dorsal and medial, while at the lower lumbar level it is more anterior and lateral (4).
Access by transforaminal route is the most widely used (4). The patient is placed in prone position with an abdominal pillow to reverse physiological lordosis. The most commonly used RF needles or cannulas are 20 or 22 G diameter and 98 mm long with 0.5 or 1 cm active tip. Following asepsis with alcoholic chlorhexidine and placement of sterile fields, radiological approaches in anteroposterior, oblique and lateral incidence are performed. In anteroposterior incidence and moving the arc in cranio-caudal direction, the double arc of the lower vertebral edge is deleted. In oblique incidence between 20 and 30 degrees ipsilateral to the DRG to be treated, the classic image described as “Scotty Dog” will be displayed, which is the result of bringing the articular facets and the spinous process closer to the contralateral side. The entry point will then be immediately below the pedicle. After local anesthesia with 1 % lidocaine, the needle will be inserted following a tunnel vision and will not advance beyond half of the pedicle in this projection to prevent neural injury. In lateral projection, it will be inserted into the roof of the neuroforamen (Figures 1 and 2) but the final location will be determined by sensory-motor nerve stimulation. Sensory stimulation is to cause paresthesia or pain in the affected territory with stimulation between 0.3-0.6 v. During motor stimulation fasciculations can be caused in the affected territory with a voltage of twice that necessary to cause paresthesia. If an intraganglionar denervation, promulgated by some authors, is desired, both sensory and motor stimulation will be positive at less than 0.3 v. As this is a more painful procedure, it is advised to administer local anesthetics after the painful stimulus and before treatment. The impedance should be kept below 450 ohms, which is achieved by infusing saline before proceeding with RF (4). The use of contrast is good practice, as it rules out the intravascular and intrathecal position of the radiofrequency cannula.

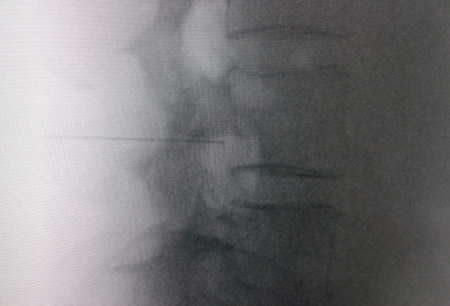
Fig. 1. Lateral radiological view showing a radiofrequency cannula at the anterosuperior angle or roof of the neuroforamen, approximate topography of the dorsal root ganglion
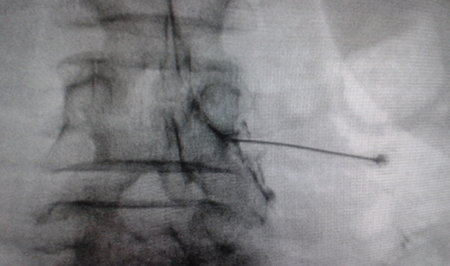
Fig. 2. Anteroposterior radiological view of a radiofrequency cannula placed in neuroforamen L4-L5, in the so-called safety triangle, after contrast injection. A thickening of the radiculogram is observed at the roof of the neuroforamen, which may correspond to the dorsal root ganglion of L4
DRG prf efficacy: evaluation of the evidence
In the present literature search, eight prospective randomized studies (23,26-32) and nine quasi-experimental studies (33-41), of before-and-after type, were found evaluating the efficacy of PRF of the DRG. It should be noted that, in the former, there is high clinical heterogeneity in relation to the control group used as a comparator, and to other interventions used before and after the evaluated procedure (e.g., steroids or local epidural anesthetics) (Table I). This heterogenicity makes difficult to interpret results and prevents the proper conduct of a meta-analysis (3). Table II shows the parameters used in the application of pulsed radiofrequency in the eight randomized prospective studies.
Table I. Randomized controlled studies to measure efficacy and safety profile of PRF of the DRG
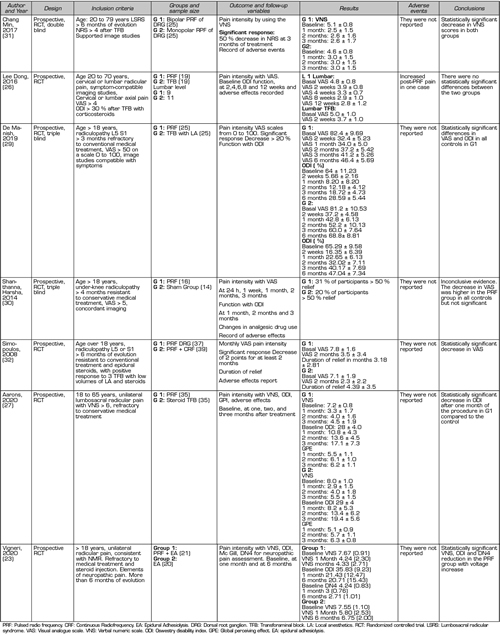
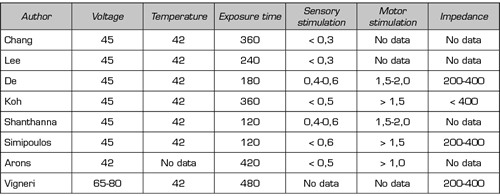
Table II. Parameters used in the treatment of pulsed radiofrequency of the dorsal root ganglion in the eight randomized studies analyzed
Lee et al. (2016) compared the effects of PRF of the DRG with those of transforaminal steroid injection in 20 patients with lumbosacral radicular pain divided into 2 groups of 9 and 11 patients, respectively (26). They use the Visual Analogue Scale (VAS) and the Oswestry Disability Index (ODI) as evaluators of results over a three-month period, finding an improvement in these scales when compared with baseline measurements, but without finding statistically significant differences between the procedures evaluated (26).
Arons et al. (2020) also use transforaminal steroid injection as a control group to assess the analgesic effect in PRF of DRG in patients with unilateral lumbosacral radicular pain (27). The authors conducted a randomized, double-blind, controlled study in 70 patients divided into two groups of 35, using the PRF of the DRG for 180 seconds in the study group. The studied variables were the global perceiving effect (GPE), The Verbal Numeric Scale (VNS) and the ODI, evaluated at 30, 60, and 180 days. The procedures were repeated at 30 days in those patients in whom the improvement was below 50 % in relation to baseline values. The authors found a larger decrease in disability in the group treated with PRF of the DRG after 30 days compared with the steroid injection group. Whereas, at 180 days, the percentage of patients with pain improvement was slightly higher in this group than in patients treated with PRF (67 % versus 60 %). No complications were reported in this study (27).
Koh et al. (2015) studied the effects of adding PRF of DGR to transforaminal epidural steroid injection in 62 patients with lumbosacral radicular pain refractory to non-interventional treatments in two study groups of 31 patients each (27). In both groups, the injection of 20 mg of triamcinolone was conducted after 3 cycles of 120 seconds of PRF or a simulation of it (sham group). Although the authors found improvement in all the parameters evaluated (VNS, GPE, ODI) when comparing the results obtained after treatment with baseline measurements, they found no statistically significant differences when comparing both groups during the 3-month control period. However, if the baseline results obtained with VNS were adjusted to zero, the decrease obtained at 3 months was higher in the group where the PRF of the DRG was performed (p = 0.038) (28).
De et al. (2019) conducted a randomized prospective triple-blind study, using transforaminal injection of 1 ml of bupivacaine 0.5 % as an active control group and PRF of DRG in three cycles for 180 seconds as a study group (29). Fifty patients with lumbosacral radicular pain refractory to conservative treatment (pharmacological, physical therapy), with a positive response prior to a selective prognostic block, were equally distributed in the two established groups. The VAS and ODI were used as outcome assessment parameters at two weeks of the procedure and at 1, 2, 3 and 6 months. One hundred percent of patients in the PRF group had a decrease of more than or equal to 2 points in VAS and a significant decrease in the ODI at all evaluation intervals. In the control group, 80 % of patients showed a decrease at 3 months and only 28 % at 6 months. The authors concluded that the 180-second application of PRF in the DRG results in a mid-term improvement of pain intensity and functional status of patients with lumbosacral radicular pain, when the previous performance of a selective prognostic block with local anesthetics is positive, discarding non-responsive patients (29).
Shanthanna et al. (2014) conducted a double-blind prospective study, using as a control group a simulation of the procedure or sham group, in which radiofrequency cannulas were placed in the neuroforamina, even with sensory and motor stimulation, but without finally performing the PRF, constituting a true placebo group (30). In the study group, a cycle of PRF was performed for 120 seconds, using 0.5 cm active tip needles. The authors found a decrease in VAS at all evaluation intervals (1 day, 1, 2, and 3 months) but did not reach statistical significance between groups. In this study, the number of patients was low, 16 in the PRF group and 15 in the control group, which required 15 months to obtain recruitment. Due to the small difference in the percentage of patients experiencing a 50 % or larger decrease in VAS between the groups, the authors decided to discontinue the study because of the excessive time required to complete recruitment, according to their judgment. In this study, no previous selection was performed based on the response to selective blocks with local anesthetics (30).
Vigneri (2020) randomized two groups of patients: PRF of the DRG was performed in 21 patients for 240 seconds and increased the voltage between 65 and 80 and then hyaluronidase, local anesthetics,
and betamethasone were administered while the control group received only adhesiolysis and PRF was simulated (23). The group in which the PRF was performed showed a significant decrease in the Visual Numerical Scale at 1 month and 6 months after treatment. Although the increase in voltage could have a positive impact on the final analgesic result, further studies are needed with more patients and with a design that
allows the performance of the increase in voltage to be assessed as the only variable (23).
Tortora (2021) published a prospective before-and-after study, with a series of 30 patients with lumbar radicular pain in which PRF of the DRG was performed with the usual parameters and controls them at one month after the intervention, seeing a significant reduction in the VAS (33). A decrease in the size of the DRG measured by nuclear magnetic resonance was found in 17 patients, compared to baseline size. This finding should be correlated with anatomopathological changes and with the intimate mechanism of action of PRF in future studies, as well as whether it has predictive value in terms of efficacy (33).
Other authors, such as Dworking et al., understand that a decrease of at least 30 % in VAS is the minimum necessary for the patient to notice a change in quality of life (42).
Rigorous patient selection is a vital requirement in the success of treatment because failure is high if the diagnosis of the source of pain is incorrect. This is why many authors recommend performing a diagnostic block with local anesthetics previously, although it is not a practice used by all centers because it significantly increases costs (43). Positive diagnostic blocks have been proposed as predictors of good prognosis for PRF of DRG, although their statistical weight is not high. Van Boxem et al. prospectively studied the prognostic weight of the following factors: Positive diagnostic block (which also impresses to be an important element in confirming clinical suspicion), age, sex, pain intensity measured by VAS, time of pain evolution, failed back surgery, disability score, and DN4 questionnaire scores (43). The authors found that age over 55 years and positive diagnostic block would be good prognostic elements, while high levels of disability would be an element of poor prognosis for treatment. However, analysis of ROC curves shows that none of these factors has a significant predictive value on its own, but that it is a combination of three factors: Positive diagnostic block, old age, and a low level of prior disability (43).
In 65 patients with lumbar radicular syndrome refractory to non-interventional treatment in whom PRF was performed at the DRG, Van Boxem et al. (2015) found a positive analgesic response in 55.4 % of patients (38). An interesting aspect is that these researchers initially planned to use a simulation or sham group as placebo control group and its implementation was rejected by an Ethics Committee, forcing researchers to modify the initial design of the study (38). In a previous study conducted by these authors, 29 % of patients who received PRF as part of their radicular pain treatment showed an improved score on a scale of 0 to 100, although with a requirement of 50 % change as a criterion of satisfactory response (39).
Castroman et al applied RF of the DRG in a group of 15 patients refractory to non-interventional treatment and repeated epidural steroid injections, finding a satisfactory reduction in pain using VNS for evaluation in approximately half of the patients selected (7 out of 15), who changed from severe pain (VNS = 8.3) to mild to moderate pain (VNS = 3.7) (40). The PRF, in addition to reducing the pain intensity, improved the interference of pain in daily activity, evaluated by the Brief Pain Inventory in its validated Spanish version (40). Recently Marliana et al. (2020) conducted a study with a quasi-experimental design, where 50 patients with lumbosacral pain syndrome were divided into two groups of 25 patients each, considering non-interventional pain treatment (pharmacological and rehabilitation) as a control group and the RF application of the RF of DRG with RF cannulas with 1 cm active tip for 120 seconds as a study group. The authors found a statistically significant decrease in VAS and ODI scores during the 4 weeks following treatment (41).
With regard to the ability of PRF in DRG to avoid surgery, there are studies that have had good analgesic results after 1 month and after 1 year of PRF (44). Trinidad et al. (2015) indicated RF of the DRG in 26 patients with radicular pain waiting for spine surgery. When the patients were evaluated after 1 year, 19 patients did not require surgery, due to the improvement obtained with the interventional procedure. An average decrease of 2.95 points in the numerical scale was observed in these patients. We note that 6 minutes of PRF were used in this study (44).
They are also retrospective studies in the literature. Abejón et al. (2007) retrospectively analyzed 54 patients with lumbosacral radicular pain treated with a total of 75 PRF procedures of the DRG, finding a statistically significant decrease in pain intensity measured by VNS, not finding the same results in patients with failed back surgery syndrome (45).
Hong et al. (2020) published a retrospective analysis of 42 treatments with PRF of DRG in patients with spinal pain, with or without radiculopathy, assessing the VNS at 1 week, 1 month, and 3 months after treatment. In this series of patients with spinal pain, PRF of DRG had a significant analgesic effect three months after it was performed, regardless of the anatomical site (cervical, thoracic or lumbar) and the presence or absence of radiculopathy. This analysis suggests that the benefit of PRF allowed the decrease in analgesic consumption, a relevant advantage for these patients (46).
In a retrospective study of 60 patients treated with PRF of the lumbosacral DRG, Kim et al. found a percentage of positive responses to treatment in about half of the patients, with better responses in those patients with positive responses to previous epidural steroid injections (47).
Finally, there are studies comparing different radiofrequency modalities applied on the DRG. Simonopulus et al. (2008) conducted a prospective randomized study in 76 patients with chronic lumbosacral radicular pain refractory to conventional treatment, clustering them into two groups: One group treated with PRF of the DRG only, and the other group treated with the same procedure followed by continuous RF (32). The authors found no statistically significant differences in the decrease in VAS scores between the groups two months after the procedures were performed. Interestingly, they found no neurological complications in the group in which continuous RF was used (32).
Sluijter et al. (1998) retrospectively analyzed a cohort of patients with lumbosacral radicular pain treated with PRF of the DRG versus continuous RF, finding at six weeks a larger number of patients with satisfactory responses on the GPE Scale in the group treated with PRF (1).
Chang et al. (2017) conducted a prospective randomized study to compare the PRF modality of unipolar (single-cannula) versus bipolar DRG, by placing two RF cannulas near the DRG (31). The recruited patients had lumbosacral radicular pain refractory to transforaminal steroid injection. The authors found a decrease in pain intensity measured by VNS at one, two and three months of the procedures, with a larger decrease in pain in the group treated with bipolar modality (p = 0.037). A production of higher intensity and denser electric fields in this group is suggested as an explanation for the larger analgesic effect (31).
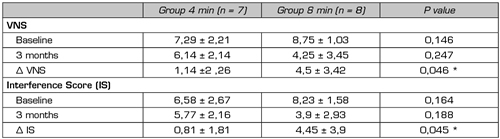
We did not find any studies comparing different schemes of exposure to PRF. In our department, a prospective, randomized, double-blind study is currently being conducted in patients with lumbosacral radicular pain refractory to epidural steroids, using as a control group the application of the exposure time to PRF that we consider as standard, that is, 2 cycles of 120 seconds (4 minutes) and as a study group, the application of 4 cycles of 120 seconds each (8 minutes). Preliminary results of the 15 patients performed (8 patients in the 8-minute group and 7 patients in the control group) show a decrease in VNS and pain interference in daily activities in both groups, being the differences between baseline and at 3-month assessments larger in the 8-minute group. Despite the low sample size, these differences reached statistical significance (Surbano and Castroman, unpublished data) (Table III). This would indicate a more powerful analgesic effect of PRF when applied for longer periods, this effect could be maintained for at least 3 months after the single application of the procedure.
In a recent systematic review of the efficacy and safety of PRF of DRG in patients with neuropathic pain, Vuka et al. (2020) concluded that, although four randomized prospective studies have been found to date, they use different control groups and there are differences in selection criteria, such as prior use of epidural steroid injections or selective blocks with local anesthetics, in addition to an insufficiently large number of patients studied, all that determines methodological defects and a high risk of bias, classifying the level of evidence as very low. A meta-analysis under these conditions was not possible according to the authors (3).
Table III. Assessment of pain intensity with the verbal numeric scale (VNS) and pain interference in daily activities (Interference Score, IS) using Brief Pain Inventory in patients with unilateral lumbosacral radicular pain, in whom it was applied, in a randomized manner, 4 or 8 minutes of pulsed radiofrequency of the dorsal root ganglion. The evaluation was performed at baseline and at 3 months, differences between baseline values and at 3 months (Δ VNS and Δ IS) were also presented
Complications
PRF is a method with a high safety profile, without major risks and with a high level of patient satisfaction (2,3). Minor complications have been described, such as pain during cannula placement, subsequent increase in radicular pain or lower back pain, headaches, or postoperative discomfort (2,3). The root puncture accompanied or not by neuritis has been described, which appears to be secondary to mechanical aggression caused by the needle rather than by the application of RF itself. Other reported complications include local erythema and pain at the needle insertion site (2,3).
Infectious complications, such as epidural abscesses, meningitis, and sepsis, have been reported in other categories of spinal interventional procedures, and therefore may potentially occur in DRG RF (48).
DISCUSSION
The effects of PRF of the DRG for the treatment of lumbosacral radicular pain have been reported in many studies. Although this treatment appears to be effective and safe for its use in patients with lumbosacral radicular pain refractory to conventional treatments, these studies have methodological issues.
In this sense, the definition of the type of control intervention is a point that generates discussions. While establishing a placebo group would appear to be the most appropriate from a methodological point of view for the design of clinical trials, at least in terms of interventional pain treatments, it generates disadvantages of different types, especially ethical. To establish a group of patients in which a simulation of a RF procedure is performed in the DRG that involves placing a needle in the neuroforamen in order not to administer nothing, seems to us to be ethically unacceptable.
Choosing a homogeneous active control group for different clinical trials seems to be a wise decision. The choice of the most suitable active control is also debatable. Non-particulate transforaminal steroids such as dexamethasone may be considered. The evidence available to date for the interventional treatment of radicular pain places epidural steroids as first-line. In our setting, the decision to use PRF of the DRG was made after the failure of two epidural steroid injections (transforaminal or interlaminar) (40). In this context, a control group with steroids would also not be the most appropriate, as we would know beforehand that the control group will not be effectively active. We therefore favor active controls with 0.5 % bupivacaine by transforaminal route, as proposed by De et al (29). It could also be lidocaine 1 % as Manchicanti uses in several clinical trials on interventional pain medicine (49,50).
With regard to the tools for assessing treatment outcomes, there seems to be considerable consensus on what to use. Most studies use a pain intensity measuring tools (VAS, VNS) combined with a disability assessment tool (ODI). In our service we have experience in using the “Inventario Abreviado de Dolor”, a validated Spanish version of the Brief Pain Inventory, as an outcome assessment tool (51). This is a useful tool for this purpose, which addresses in a single-form aspects related to the intensity of the pain and interference of the pain on important aspects of the active and emotional life of the patients, so we consider it a complete form of evaluation. Furthermore, question number 6 of the questionnaire refers to the intensity of pain at the time of application of the questionnaire, which may be equivalent to the application of the verbal numeric scale (VNS) (51).
There is also some discrepancy between the authors regarding what change should occur between baseline values and controls to consider an analgesic response acceptable. We consider a change of at least 30 % from baseline acceptable, considering that this percentage of change is considered as a satisfactory minimum response for interventional procedures on the spine (52).
In this review, several studies were found with quasi-experimental design, before-and-after type, or cohort studies. These studies, while providing information of interest, do not eliminate the bias of the placebo effect to a new treatment option or that inherent in the spontaneous resolution of symptoms because no control group is included in them. However, studies on the use of PRF of the DRG have been conducted in patients with chronic lumbosacral radicular pain, with progression times larger than 3 months. Chronic radicular pain has little chance of spontaneous improvement beyond these times.
Another point at which progress is needed is to homogenize patient selection criteria. The analysis of the studies shows that there is a variable percentage of patients who do not respond to PRF of the DRG. The positive response of a prognostic selective block with lidocaine as an inclusion criterion may help to rule out non-responsive patients beforehand, at least partially. However, such strict selection criteria conspire against the recruitment process, which can lead to lengthy studies over time to achieve adequate sample sizes.
Moreover, studies addressing the effect of the different variables involved in the technique (e.g., active tip and cannula thickness, exposure time, etc.) on the result of the technique are necessary to allow the treatment to be protocolized. In this same sense, it is necessary to establish the spinal segments to be treated, considering the abundant metameric divergence of nociceptive afferents. The value of perform repited PRF-DRG procedures, in the understanding that the neuronal changes obtained are transient, is also another element to be studied.
Finally, we understand that beyond the current relative weight of the evidence, in the absence of alarm elements requiring surgery, the treatment of radicular pain with RF of the DRG should be offered prior to proposing surgical treatment.
CONCLUSIONS
The PRF of the DRG is outlined as a useful procedure for the treatment of lumbosacral radicular pain, improving the intensity of pain and the disability generated by it when standard, interventional and non-interventional treatments fail to achieve satisfactory responses. Despite this, the evidence available to date should be considered weak and preliminary, findings obtained should be confirmed with high-quality randomized prospective studies, with a higher number of participants, homogeneous selection criteria and homogeneous control groups.
REFERENCES