
DOI: 10.20986/resed.2020.3747/2019
ORIGINAL
Effectiveness of patient-controlled analgesia in acute and chronic pain after cardiac surgery: a prospective study
Efectividad de la analgesia controlada por el paciente en el dolor agudo y crónico después de cirugía cardiaca: estudio prospectivo
N. Esteve-Pérez1,2, J. Iborra-Escalona1, G. Gómez-Romero1,2, C. Sansaloni-Perelló1, A. M. Verger-Bennasar1, S. Tejada-Gavela3, M. Riera-Sagrera4 and L. C. Mora-Fernández1,2,5
1Servicio de Anestesia, Reanimación y Terapia del Dolor. Hospital Universitario Son Espases. Palma de Mallorca, España. 2Instituto de Investigación Sanitaria de las Islas Baleares (IdISBA), Palma de Mallorca, España. 3Departamento de Biología de la Universidad de las Islas Baleares. CIBEROBN (Physiopathology of Obesity and Nutrition), Palma de Mallorca, España. 4Jefe de Sección de la Unidad de Cuidados Intensivos. Hospital Universitario Son Espases. Palma de Mallorca, España. 5Jefe de Servicio de Anestesia, Reanimación y Terapia del Dolor. Hospital Universitario Son Espases. Palma de Mallorca, España
Received: 02-06-2019
Accepted: 02-01-2020
Correspondence: Neus Esteve-Pérez
neus.esteve@ssib.es
ABSTRACT
Introduction: The intensity of postoperative moderate/intense pain after cardiac surgery (CC), varies according to the different studies, from 45 % to 85 %. There is no evidence about which is the optimal analgesic regimen in the postoperative period.
The main objective of this study is to evaluate the effectiveness of the application of a multimodal analgesic protocol, based on patient-controlled analgesia (PCA) with morphine, in patients undergoing cardiac surgery with extracorporeal circulation (ECC).
Patients and methods: Prospective observational study of all patients undergoing CS with ECC, during the first 3 days postoperatively. There were included 102 patients in two periods, first, in November 2016 with conventional analgesia and second, in January - February 2017 with PCA.
Results: The pain at rest was controlled (median numerical scale <3). An average of 27 % of moderate and intense pain was recorded. There was no difference in pain intensity between patients with CA and those with PCA. The PCA group required less rescue analgesia in the first postoperative days (63 % vs. 44 % p = 0.0487). The incidence of Post-surgical Chronic Pain was 39 % at three months, and 3 % at one year. There was a correlation between preoperative pain and anxiety with the intensity of the dynamic pain (r = 0.287, p = 0.03).
Conclusions: PCA with on-demand opioids and multimodal analgesia is an effective alternative after cardiac surgery. Good control of postoperative pain is obtained without increasing adverse effects, and requiring less rescue analgesia administered by the nursing staff.
Key words: Postoperative Analgesia, cardiac surgery, multimodal analgesia, patient controlled analgesia (PCA).
RESUMEN
Introducción: La intensidad del dolor postoperatorio moderado-intenso después de una cirugía cardiaca (CC) varía, según los estudios, de un 45 a un 85 %. No existe evidencia sobre cuál es la pauta analgésica óptima en el postoperatorio de estos pacientes.
El objetivo de este estudio es evaluar la efectividad de la aplicación de un protocolo analgésico multimodal, basado en la analgesia controlada por el paciente (PCA) con morfina, en pacientes sometidos a CC con circulación extracorpórea (CEC).
Pacientes y métodos: Estudio prospectivo de todos los pacientes sometidos a CC con CEC, durante los primeros 3 días del postoperatorio (DPO). Se incluyeron 102 pacientes en dos periodos, noviembre de 2016, con analgesia convencional (AC) y enero-febrero de 2017 con PCA.
Resultados: El dolor en reposo se mantuvo controlado (mediana escala numérica < 3). Se registró un 27 % de pacientes con dolor moderado e intenso. No hubo diferencias en la intensidad del dolor entre los pacientes con AC y los de PCA. El grupo de PCA precisó menos analgesia de rescate (63 vs. 44 %, p = 0,0487). La incidencia de dolor crónico postquirúrgico fue de un 39 % a los tres meses y un 3 % al año. El dolor y la ansiedad preoperatorios se correlacionaron con la intensidad del dolor dinámico (r = 0,287, p = 0,03).
Conclusiones: La PCA con opioides a demanda y analgesia multimodal es una alternativa efectiva después de la CC. Se obtiene un buen control del dolor postoperatorio sin incrementar los efectos adversos y precisando menos analgesia de rescate administrada por enfermería.
Palabras clave: Analgesia postoperatoria, cirugía cardiaca, analgesia multimodal, analgesia controlada por el paciente (PCA).
INTRODUCTION
The effective control of acute postoperative pain (POP) has become an essential part of perioperative care. Its appropriate management, together with other factors such as patient mobilization and early nutrition, is related to a decrease in postoperative complications and length of hospital stay (1). POP has been associated to an increase in morbidity and costs (2), a decrease in patient comfort, and a higher risk of chronic pain development (3).
POP after cardiac surgery (CS) is described as a moderate to intense pain, with an important potential pathophysiological impact. The highest pain level appears during the first two postoperative days (POD), starting to decrease at the third day after surgery and achieving mild levels from the first week on (4). Pain origin is located in several anatomic areas: sternotomy, sternal/rib retraction, pericardiotomy, internal thoracic artery harvesting, saphenous vein harvesting, surgical manipulation of the parietal pleura, chest tube insertion (mediastinal and pleural), central venous or arterial catheter cannulation, and other musculoskeletal trauma (5).
The prevalence of POP in CS varies widely across studies. Navarro (6) et al. report a 45% of patients with moderate to intense pain in the first 24 hours, whereas this percentage increases to 70% for Keawnantawat (7) et al. and to 85% for Bjørnnes (8) et al. Studies are very heterogeneous and it is difficult to add results in order to obtain valid conclusions. However, authors agree on the high incidence of moderate to intense pain in CS and on the fact that analgesic treatments currently prescribed are probably insufficient. On the other hand, there is no conclusive evidence on which is the optimal analgesic regimen in the postoperative phase of these patients (9).
The main objective of this study is to evaluate the effectiveness of applying a multimodal analgesic protocol based on patient-controlled analgesia (PCA) with intravenous morphine during the first 3 PODs in patients undergoing CS with extracorporeal circulation (ECC). We have related pain intensity to demographic variables, the comorbidity of patients, intra and postoperative data, as well as with the presence of chronic postsurgical pain (CPSP) at one year after surgery.
MATERIAL AND METHODS
Design
Prospective, observational study of all patients undergoing CS with ECC during their first 3 PODs in the intensive care unit (ICU) and the general care floor. The study is based on interviews to and the record review of all patients treated before and after implementing a multimodal perioperative analgesic protocol based on patient-controlled analgesia with morphine.
The study was approved by the Research Ethic Committee of the Balearic Islands (No. IB 3376/16 PI) and follows the Helsinki Declaration recommendations on biomedical research. All patients were informed about the study protocol before signing the specific informed consent.
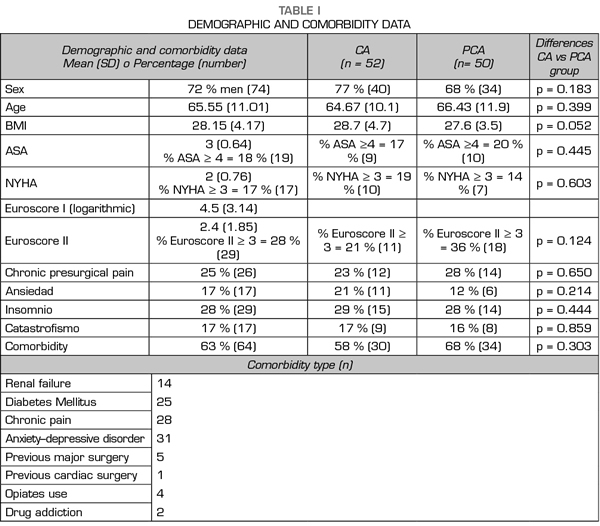
Study population: We included all consecutive patients of two periods: a first group in November 2016, treated with conventional analgesia (CA group), and a second one in January to February 2017, treated with patient-controlled analgesia (PCA group).
Inclusion criteria were age >18, acceptance of participation in the study and signature of the informed consent, as well as elective CS with ECC pump and subsequent ICU admission. Exclusion criteria were under age patients (< 18), non-acceptance of participation in the study, patients with cognitive impairment or verbal communication impossibility, or patients receiving invasive mechanical ventilation for more than 24 hours after surgery.
Procedures
Patients underwent elective or emergency coronary surgery, valvular surgery, or both. The surgical protocol included either median sternotomy, thoracotomy or minimally invasive approach; extracorporeal circulation with moderate hypothermia (32 °C); and harvesting of the internal thoracic artery and/or the saphenous vein.
The anaesthetic protocol included ventilatory and invasive hemodynamic monitoring, as well as anaesthetic depth control. Non-invasive cerebral oximetry and transesophageal echocardiography were applied as indicated by the anaesthesiologist. Midazolam, fentanyl and propofol were used for induction. For maintenance, patients received sevoflurane or propofol, depending on the anaesthesiologist’s preferences. Intraoperative analgesia was administered with on-demand fentanyl boluses (total dose 10-15 µg kg-1) or remifentanil intravenous (IV) infusion (dose < 0.2 µg kg-1 min-1). Muscular relaxation was achieved with rocuronium or cisatracurium. Preventive analgesia was applied 30 minutes before exiting the operating room using dexketoprofen (50 mgs) or dipyrone (2 grs), paracetamol (1 gr), dexamethasone (8 mgs) and tramadol (100 mgs) or morphine (5 mgs). Patients were transferred to the ICU and extubated on fulfilment of the hemodynamic and respiratory stability criteria.
For the CA group, a dexketoprofen (50 mgs / 8 h) or dipyrone (2 grs / 8 h), and paracetamol (1 g / 6 h) regimen with 5 mg morphine boluses was prescribed as rescue analgesia, when the numerical rate scale (NRS) > 4, after extubation.
Intervention
For the PCA group, the indicated regimen after extubation included an on-demand morphine PCA pump (1 mg/1 ml) with 1 mg boluses, 10 min lockout intervals and a maximum of 6 boluses / hour. Additionally, a dexketoprofen (50 mgs/8 h) or dipyrone (2 grs/8 h), and paracetamol (1 g/6 h) regimen with 5 mg morphine boluses was prescribed as rescue analgesia, when the NRS > 4, after extubation. The pump was removed at 48 hours after surgery if the pain was controlled (NRS < 4) and the patient pump usage was < 10 boluses/24 hours.
Data collection
Patients included in the study answered to three different surveys: a preoperative survey, a postoperative of POP survey in the first 3 PODs, and a telephonic CPSP survey at 3, 6 and 12 months after surgery. We also collected data from the medical records and added it to the survey information.
The patients surveys were carried out by three members of the study team trained for postoperative pain assessment.
With the preoperative survey, adapted from the Brief Pain Inventory (10) (BPI) (Appendix A), we explored the presence of pain, anxiety, depression and catastrophic thinking.
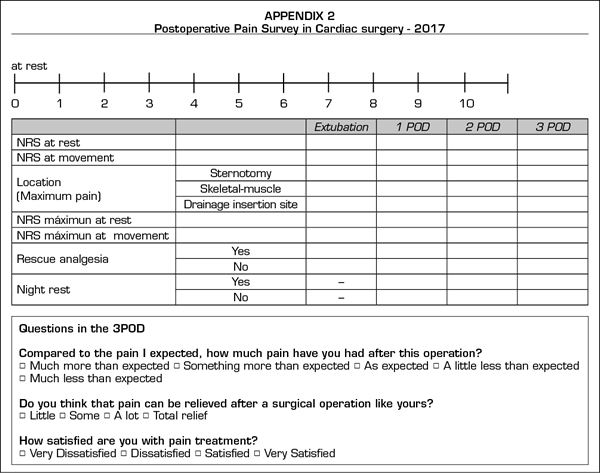
The postoperative POP survey (Appendix B) is adapted from the questionnaire by the American Pain Society (11). We registered pain intensity at rest and on activity using the numerical rate scale (NRS 0 to 10, 0 = no pain, 10 = maximum pain). Patients were asked to communicate the pain level at the time of the interview (observed pain) as well as the maximum intensity of pain experienced during the postoperative period (maximum pain experienced). We then classified patients according to the pain level stated: absence of pain (NRS < 3), moderate pain (NRS 4-6), intense pain (NRS > 6) and any pain level (NRS > 4). Other data collected was the pain origin localization in three specific areas (sternotomy, drainage insertion site and skeletal-muscle pain), the use of rescue analgesia, possible adverse effects of analgesics, and nocturnal rest degree.
Finally, we asked patients about their expectations and the reality of the experienced pain, as well as about their satisfaction with the administered analgesic therapy.
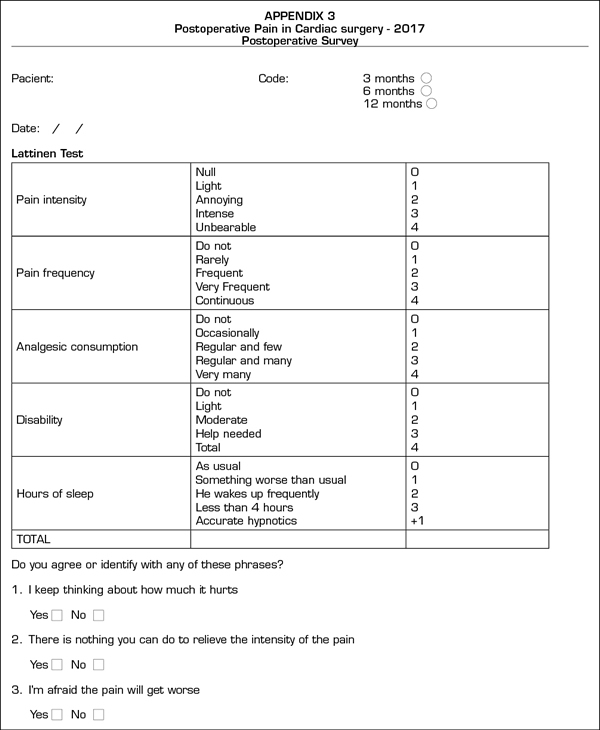
The postoperative CPSP survey (Appendix C) is based on the Lattinen Test (12) on chronic pain. In this phase, we repeated the questions regarding catastrophic thinking already raised in the preoperative survey.


Variables
We defined demographic variables, the ASA score (American Society of Anesthesiologists), the NYHA scale (New York Heart Association) and version I and II of EUROSCORE (European System for Cardiac Operative Risk Evaluation).
The following conditions were defined as comorbidity variables: diabetes mellitus, renal failure risk (creatinine > 1.2), chronic pain (pain for more than 3 months at any location with chronic analgesic consumption), anxiety–depressive disorder (chronic consumption of benzodiazepine or psychotropic drugs), previous major surgery, previous cardiac surgery and opioids or drugs use.
Perioperative variables registered were the type of cardiac surgery, surgical approach, drainage type, CS duration and ECC, maintenance anaesthetic and analgesic management (propofol, sevoflurane, remifentanil, fentanyl or morphine), preventive analgesia administration, prescribed analgesia during the first 3 PODs (intravenous or oral), and rescue analgesia.
During the CPSP survey, the postoperative variables recorded were pain intensity and frequency (0 to 4), analgesics usage (0 to 4), functional capacity (0 to 4) and sleep quality (0 to 4).
Sample size
After reviewing the existing literature on analgesic effectiveness, we defined it as a 20% (13) reduction of NRS for the PCA group. With a statistical power of 0.8 and a significance level of 0.05, the minimum sample size of 26 patients per group.
Statistical analysis
We carried out the statistical analysis using the software package SPSS, version 24.0. Qualitative variables were described using absolute number and percentages. Quantitative variables were described using mean values and standard deviation or median and interquartile range (IQR Q1-Q3). In order to compare the patients’ characteristics depending on the analgesic regimen and the identification of pain level related variables, the Mann-Whitney U test and the chi-square test or the Fisher’s exact test were applied for quantitative and qualitative variables, respectively. We considered values of p < 0.05 statistically significant.
RESULTS
A total of 102 patients were assessed: 52 receiving CA and 50 with PCA. Figure 1 presents a flow chart of the study protocol and the excluded patients. Tables I and II present demographical, comorbidity and intraoperative data. No differences were observed in demographical data, comorbidity, risk scales or surgery type among groups. No deceases were registered during the study.
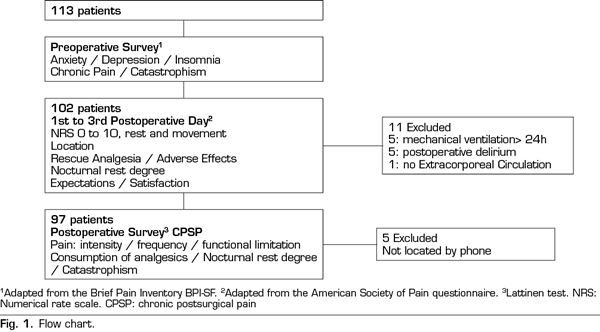
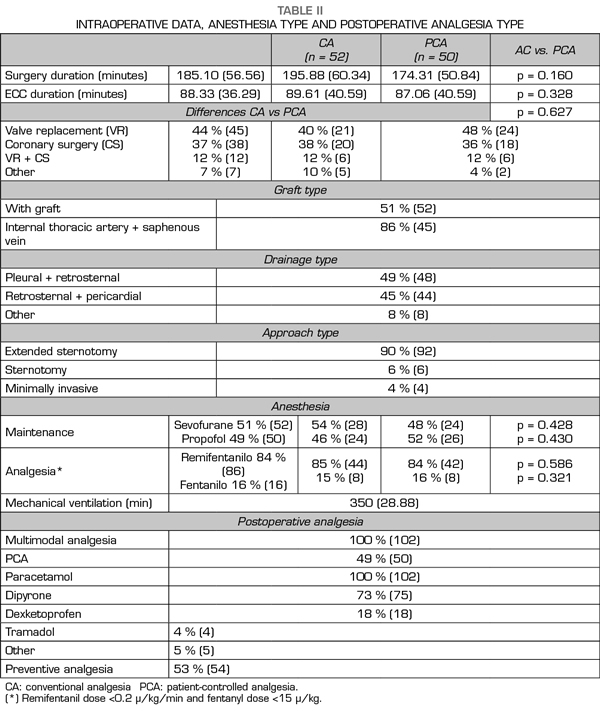
Pain at rest was controlled (median NRS < 3), whereas moderate and intense pain on movement was registered from extubation (median NRS 5, IQR 2-8) to the third POD (median NRS 5, IQR 3-7). A great interindividual variability was observed, with a maximum and minimum range of 10 and 0, respectively. The percentage of patients with controlled pain at rest was 68% at extubation and 85% on the third POD. The registered average of patients with moderate and intense pain at rest was 27%, of which 20% was moderate and 9% was intense, with an evolution from 19% and 14% at extubation to 13% and 4% on the third POD, respectively. Registered pain at movement was 34% for moderate and 38% for intense pain. The maximum pain at rest reported by patients was kept under NRS 3, with 44% and 25% of patients with maximum moderate pain at rest on the first and the third POD, respectively.
Figure 2 presents the pain evolution from extubation to the third POD. Figure 3 presents the evolution of maximum pain reported by patient.
Pain at extubation and during the first 3 PODs was identified with the sternotomy location in 89% of the cases, and with a skeletal-muscle origin or the drainages by less patients (9% and 2%, respectively).
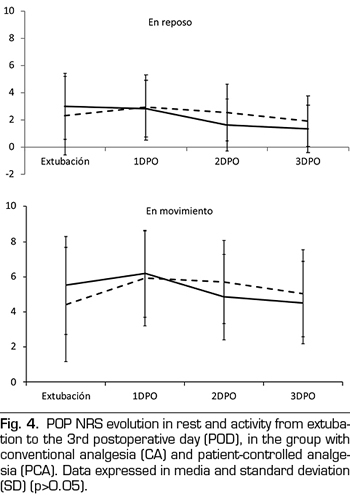
No differences in pain intensity between CA or PCA patients was observed during the first three PODs, either at rest or on mobility (Figure 4).
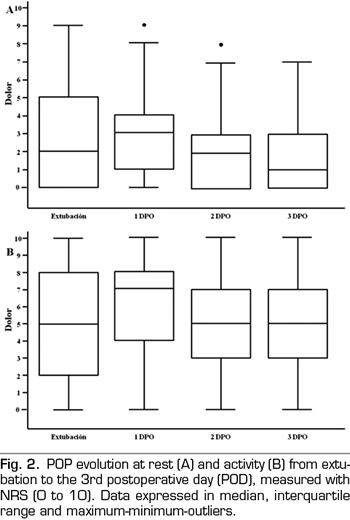
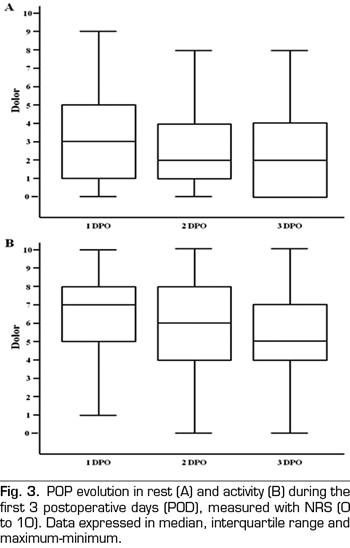
Rescue analgesia was necessary for 54% of patients at extubation, 54% on the first POD, and 10% and 1% on the second and third POD, respectively. The PCA group needed less rescue analgesia on the first POD than the group with conventional analgesia (63% vs. 44%, p = 0.0487), as well as less morphine (1.9 mg vs. 4.5 mg per patient). Analgesia conversion to oral intake was performed on the third POD in 92% of PCA patients and in 46% of AC patients (p < 0.001).
Regarding nocturnal rest, 40% of patients were not able to get to sleep on the first POD, and neither were 34% and 25% on the second and third POD, respectively. No differences were observed among groups.
More pain as expected was reported by 35% of patients, without differences among groups (PCA 35% vs. CA 36%, p = 0.788). The analgesic therapy received was perceived as satisfactory by 95% of patients. No differences were observed among groups (PCA 98% vs. CA 92%, p = 0.183).
Analgesia-related adverse effects were registered in 7% of patients (3 patients with nausea/vomiting, 3 with delirium and 1 with postoperative ileus). No significant differences were observed among groups (PCA 4% vs. CA 10%, p = 0.262).
No differences were noted in the average length of ICU stay (2.2 vs. 2.2 days) or hospital stay (PCA 10.5 vs. CA 10.7 days).
No differences were registered in pain intensity for patients receiving remilfentanil (84%) or fentanyl (16%) (p = 0.201).
CPSP incidence was 39% at three months after surgery, 11% at six months, and 3% at one year. Percentage of patients experimenting intense CPSP was 9%, 1% and 1%, respectively. No significant differences were observed among groups at three months (PCA 44% vs. CA 37%, p = 0.533).
At three months after surgery, 2% of patients with CPSP informed of a regularly intake of analgesics and a significant functional limitation, and 14% reported insomnia and catastrophic thinking. At one year, no patient was regularly using analgesia nor had any pain-related functional limitation, 3% of patients were using hypnotics and 1% presented a catastrophic profile.
POP intensity was negatively correlated with age, especially on the second POD (r = -0.311, p = 0.01). A positive correlation was registered between dynamic pain, insomnia, anxiety and presence of preoperative pain (r = 0.287, p = 0.03). Catastrophic thinking was related with a higher intensity of POP, especially on the third POD (r = 0.285, p = 0.04). No correlation was found between POP and sex or surgery type and duration.
DISCUSSION
Data of the present study show a pain at rest median below NRS 3 for all patients. However, 20% of patients experienced moderate pain and 9% intense pain at rest. Registered pain at movement was 34% for moderate and 38% for intense pain. These data confirm the fact that average pain values of surgical interventions do not properly describe the effectiveness of analgesic therapy.
Dynamic pain was always moderate to intense. Control of pain on activity in CS is essential to ensure early mobilization and respiratory physiotherapy, adrenergic stimuli blocking, proper patient comfort, and a minimization of a CPSP possibility. However, results in current surgery practice (14) question the fact that POP control should be based only on the NRS <3 goal. Early ambulation, physiotherapy or rehabilitation has been proven to be feasible options with a moderate pain level. Establishment of a NRS <3 goal without taking into account the functional impact can be associated with patient immobility or analgesic-related side effects negatively affecting prompt patient recovery. This sets out the concept of Optimal Postoperative Analgesia (15), which aims to optimize patient comfort, accelerate functional recovery and minimize side effects.
Data for pain origin at sternotomy during the first three PODs (89%) coincides with data of other studies (16), although pain at the saphenous harvesting location was registered starting with the fourth POD and the increase in ambulation.
No significant differences were registered in POP intensity among both groups. Moderate to intense pain at rest percentage (27%) is lower than data provided by other studies (45%-85%) (6,8). This means that both analgesic regimens were efficient, and that the nursery department was as efficient with the CA group as the on-demand boluses self-administered by patients of the PCA group.
PCA patients required less rescue analgesia and fewer rescue morphine doses, and regimen was converted to oral analgesia earlier.
POP perception presents a great interindividual variation. The factors that have been associated with higher POP intensity in CS are age <65, internal thoracic artery graft, preoperative anxiety (6), and catastrophic thinking (17). Besides of these factors, the present study also associated the presence of preoperative pain and insomnia with higher pain intensity. Intravenous morphine during the first three PODs is described in literature as an average of 50 ± 15 mg (18). These average values show that patients’ needs may largely vary. Rescue analgesia, on the other hand, is described for 65% of patients (19). This percentage is similar to the number of CA patients in the present study requiring rescue analgesia, and superior than the analgesic demands of PCA patients (63% vs 44%, respectively; p=0.0487). These data highlight the need of prescribing rescue analgesia together with the baseline analgesic regimen in order to provide a patient-tailored therapy. Other factors that may modify analgesic needs and that must therefore be taken into account when planning postoperative analgesia are previous use of opioids, surgical reintervention, extended surgery duration, anxiety–depressive disorder, or another associated surgical intervention.
Detecting patients with a higher risk of intense postsurgical pain during the preoperative phase would allow anaesthetists to apply specific therapeutic measures such as continuous PCA, local analgesia or adjuvant drugs to boost analgesics effects.
IV opioids are the most effective analgesic in moderate to intense pain management. However, opioid-sparing regimes are currently used to minimize their potential adverse effects and accelerate postoperative recovery. The most effective administration method is PCA (5,20,21), which it is proven to reduce morphine use, improve pain control and adapt to individual patient needs, at the same time that reduces nursing staff working load. Notwithstanding, the present study did not register differences between PCA and CA with on-demand doses administered by the nursing staff. The systematic review by Nachiyunde B (21) et al. also presents different results from different studies. This fact is based on determinant factors such as nursery staff training on analgesics, the nurse-patient ratio, and the existence of specific analgesic protocols8. On the other hand, PCA effectiveness (22) is greater in surgeries with a superior intense pain at rest such as major open abdominal surgery or thoracotomy.
No differences were registered for patient satisfaction depending on the analgesia type. Although the satisfaction percentage was higher in the PCA group, no significant differences were observed (PCA 98% vs CA 92%, p = 0.183). This is a complex indicator, also related with patient expectation and previous experiences. Therefore, high satisfaction values may be reported despite poor pain management.
Multimodal analgesia has been proven to reduce opioids use and its side effects (5). In the present study, 100% of patients received multimodal analgesia, with the administration of paracetamol to 100% of patients, metamizol to 73%, as well as morphine in PCA or as rescue doses.
The use of non-steroidal anti-inflammatory drugs (NSAID) is controversial in CS (23). These have been proven to be safe for patients under 70 and with normal preoperative renal function. With these patients, its use during a short period has not been associated to a greater mortality, myocardial infarction, renal failure or gastrointestinal bleeding (19,20).
Other analgesic options, effective from a multimodal point of view (23,24), have also been described in literature, but there is not enough evidence for its generalized recommendation. Alternatives used include epidural analgesia (25), pregabalin (26), oral oxycodone (27), dexmedetomidine, or regional analgesia (paravertebral (28), intercostal or parasternal (13) block).
CPSP incidence in CS has been described in literature in 11-46% of patients, although only 2-10% presented intense pain (24,29). CPSP incidence registered in the present study was of 3%, lower than other series such as that studied by de Hoogd S (30)et al. (19%) or Lahtinen P (31) et al. (16%). All studies agree that there are a low percentage of patients with intense pain and functional limitation at one year after surgery. In the present one, this was of 1% without differences between groups. It should be noted that there is a great heterogeneity both in studies and in CPSP assessment methods. Setälä P (32) et al., for instance, pointed out the difference between data registered during a call survey (38%) and during physical examination (15%).
Remifentanil IV infusion (doses <0.2 µ/kg/min) was administered to 84% of patients. The use of remifentanil has been associated to a greater POP intensity and to a higher CPSP incidence. However, data on this issue is controversial. The study by de Hoogd S (33) et al. registered a higher pain level and postoperative opioids use, as well as a greater CPSP incidence at three months, but not at one year. This may be an explanation to the contradictory results presented in different articles. Our results do not show a relation between the use of remifentanil or fentanyl and POP or CPSP. However, the fentanyl group was very reduced (16%).
No differences were observed in the average ICU or hospital stay. Notwithstanding, average stay is a limited indicator for analgesic effectiveness, as it depends on factors such as the variability of the clinical practice or the different forms of hospital organization, among others.
The limitations of this study are derived from its non-randomized observational nature and from the sequential evaluation before-after the application of the PCA analgesic protocol. Despite the homogeneity of both patients groups, bias due to lack of randomization cannot be ruled out. The findings represent experience at a single institution, limiting their generalization.
CONCLUSIONS
PCA with on-demand opioids and multimodal analgesia is an effective alternative following cardiac surgery. Good control of pain and a prompt conversion to oral analgesia are obtained without increasing adverse effects and requiring less rescue analgesia administration by the nursing staff. The analgesia type did not affect the average stay or the CPSP incidence. We registered a percentage of patients with moderate to intense pain who would need specific analgesic protocols in order to improve postsurgical results.
ACKNOWLEDGEMENTS
To the anesthesiologists section of Cardiac Surgery, to the Dr. Saez de Ibarra-Sánchez JI, Chief of Department of Cardiac Surgery and to the team of surgeons, to the Dr. Vidal-Bonet L from the Cardiology Department, to the nursing of the Post-Anesthetic recovery unit and to the Critical Care Medicine Department nursing team, whose collaboration has been indispensable for the accomplishment of this work.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
REFERENCES