DOI: 10.20986/resed.2018.3664/2018
ARTÍCULO ESPECIAL
Oxytocin and chronic pain in humans
X. Palacios-Espinosa1, A. M. Gómez-Carvajal1, J. S. Botero-Meneses2 y L. Palacios-Sánchez2
1Profesora de Psicología y 2Profesor de Neurociencia. Escuela de Medicina y Ciencias de la Salud. Universidad del Rosario. Bogotá, Colombia
Recibido: 02-02-2018
Aceptado: 05-02-2018
Correspondencia: Leonardo Palacios-Sánchez
leonardo.palacios@urosario.edu.co
ABSTRACT
Oxytocin (OT) is a long known neuropeptide, and it's uses in clinical practice, mainly those regarding obstetrics have been thoroughly studied. However, there's been an increasing interest in identifying other roles OT plays in emotion, cognition, perception and higher cognitive processing. OT is also believed o have a mayor role in the physiology and physiopathology of pain. We have reviewed existent literature and current human studies on the relationship between OT and chronic pain. The present work compiles a significant number of research papers and may cast some light on a remarkably interesting and promising matter.
Key words: Chronic pain, oxytocin, prosocial behavior, emotion, pain.
RESUMEN
La oxitocina (OT) es un neuropéptido conocido desde hace mucho tiempo, y sus usos en la práctica clínica, principalmente los relacionados con la obstetricia, se han estudiado en múltiples ocasiones. Sin embargo, ha habido un creciente interés en la identificación de otros roles que la OT pudiera tener en la emoción, la cognición, la percepción y otras funciones cognitivas superiores. También se cree que la OT es de gran importancia en la fisiología y la fisiopatología del dolor. Hemos revisado la literatura existente y los estudios actuales en humanos sobre la relación entre la OT y el dolor crónico. El presente trabajo compila un número significativo de trabajos de investigación y pudiera arrojar alguna luz sobre un asunto sumamente interesante y prometedor.
Palabras clave: Dolor crónico, oxitocina, conducta prosocial, emoción, dolor.
The aim of this paper is to present and discuss the findings available in scientific literature in the past 40 years regarding the effect of OXT in chronic pain in humans.
Oxytocin (OXT) is a nonapeptide produced in the supraoptic and paraventricular nuclei in the hypothalamus of mammals (1). Its etymology comes from ancient greek words ?ξ?ς oxys “fast” and τ?κος tokos “birth”. In 1906 British physiologist Sir. Henry H. Dale discovered that concentrate extracted from an oxen’s posterior hypophysis promotes uterine contractions in other animals when administered intravenously (2).
In 1909, William Blair Bell, a British gynecologist, was the first to use Dale’s findings in a clinical setting on human subjects. He used a concentrate from infundibular regions of women in labor, facilitating uterine contractions and preventing post-partum hemorrhaging. It also helped in the treatment of constipation in both men and women (2). In 1911 the same substance, called “infundibulin”, facilitated the production of milk in animals and in humans (2).
In the 30s of the last century it was assumed that the hormones that were in the neurohypophysis were synthesized by pituicytes of the infundibular process. American researchers of German origin, Ernst and Berta Scharrer, were the first to point out that hormones were produced in the supra-optic and paraventricular nuclei of the hypothalamus. In 1949 Bargman managed to demonstrate that axons that were projected from these nuclei were responsible for the transport of these substances to the neurohypophysis. He named these projections as the “neurosecretory pathway” (2).
Starting the decade of the 50s of the last century, the American chemist Vincent du Vigneaud identified the sequence of nine amino acids of OXT and was the first to synthesize it artificially in 1953, (3) he won the Nobel Prize in chemistry in December 1955 for have achieved the characterization and synthesis of the neuropeptide.
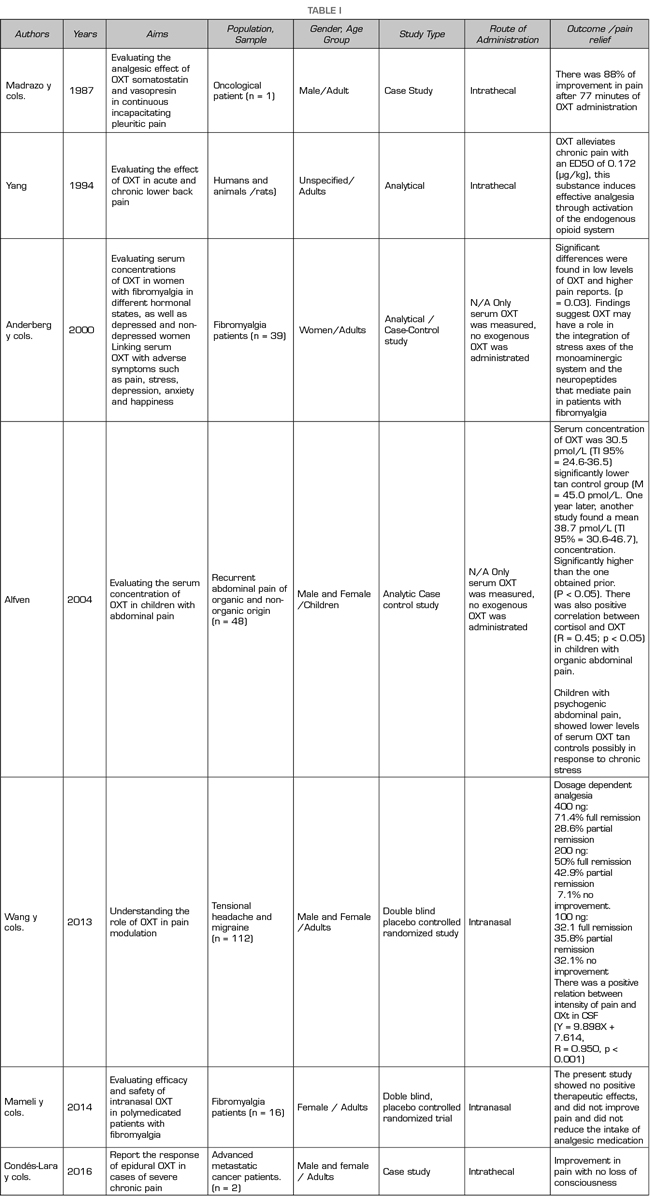
The first studies on pain treatment with OXT in humans date from 1987, when Madrazo et al., Used OXT intrathecally in a woman with mesothelioma, achieving an improvement in pain in 88% after 77 minutes of administration (4) Other studies report the use of OXT in this way for low back pain (5). OXT has also been administered as an analgesic intranasally (6-9).
In general, the analgesic use of OXT in humans has focused on chronic pain; that is, one whose duration is equal to or greater than three months, is prolonged or persistent (10), negatively impacts the general health, mood and socioeconomic well-being of the person who develops it and is considered as a disease in itself (eleven).
OXT influences higher brain functions, and although its chemical structure has not changed particularly for more than 700 million years, until now the mechanisms underlying its functions are beginning to be understood (11).
OXT works in groups of specific “OXTergic” cells that are part of what is known as the OXT system. It exerts its mechanism of action through binding with specific OXT receptors (12). Apparently, this neuropeptide mediates the regulation of emotional processes that are part of the transformation of acute pain to chronic pain (12) and is present peripherally and centrally in various concentrations, which helps explain the differences in experience painful of human and non-human animals (12).
Studies on the use of OXT in humans for analgesic purposes date back to 1987. Since then, only seven studies have focused on this effect in people with chronic pain (4,5,7,9,13-16) and between they, only three in the last 5 years (7,9,16) (Table I).

Recently, Goodin, Ness et al., Conducted a critical review of the literature on recent advances that allow a better understanding of the analgesic properties of OXT and its role in nociception (6,12). In this review, they dedicate a specific part to show evidence of the analgesic effect of OXT in humans and although they agree that studies are scarce, they conclude that OXT has a modulating potential in the perception of pain, which is supported by randomized controlled trials with controlled placebo (7,13).
Likewise, these authors indicate that low levels of OXT may be associated with a greater perception of chronic pain as it increases peripheral and central sensitization to pain. They report that the studies are heterogeneous but agree that OXT decreases sensitivity to pain. They also found that, in people with chronic pain, OXT levels are lower compared to healthy subjects (6).
Among the limitations reported by Goodin et al., are that some of the studies conducted with humans show results that are not directly comparable because the measurement or administration of OXT differ (12). Finally, they indicate that it is essential to perform studies that allow determining the differences in OXT concentrations at the peripheral and central level (plasma vs. cerebrospinal fluid), as well as the routes of administration in these two levels that differentially affect pain experiences. in humans They finalize their review of OXT and pain in humans, proposing that the use of intranasal OXT be considered standardized, since several studies have used this presentation (6).
Tracy et al. also conducted a review of the literature in which they discuss the effective applications of OXT for pain management in a healthy population and its potential use as a modulator of painful experience. They conclude that OXT seems a promising analgesic but that more empirical evidence is still needed to support its efficacy (5).
CONCLUSIONS
Certainly, the interest in understanding the function of OXT has grown exponentially, due to the potential ability to improve social skills (5), alleviate psychiatric conditions such as depression and modulate chronic pain (5).
The publications of the last five years on the subject of OXT and pain practically duplicated those of the decade 2000-2011, which suggests that interest in the topic has increased. However, the populations that have been studied are diverse, some with chronic pain, others with acute pain, others healthy, but certainly by the characteristics of their clinical pictures, the pain is not comparable and, therefore, it would hardly be the effectiveness of OXT. Studies that evaluate the analgesic effects of OXT in chronic pain are even more scarce.
Most studies on chronic pain and OXT have been performed primarily with adults; only two have included pediatric population (7). The research subjects were patients with chronic diseases such as abdominal pain, back pain, fibromyalgia, migraine, irritable bowel syndrome and cancer. Specifically, two studies have included cancer patients in the terminal phase of the disease (8).
Regarding the route of administration, in these studies for analgesia in chronic pain, intranasal and intrathecal predominate. Tracy mentions that intranasal administration induces peripheral and central effects which can be measurable (5). In general, the authors of the various studies reported analgesic effects of OXT (9,12,16). Scarcely, in one of them, the authors indicate that at least in some proportion, OXT had no analgesic effects (10).
The few reports of side effects in the reviewed studies are striking. Among them, only one indicated that the participants experienced headache (11). On the other hand, in two of the publications the authors reported that there were no side effects due to the use of this substance (12).
Taking into account diversity in the population and the experimental methods in which it was sought to observe the response in patients with chronic pain in relation to the effect of OXT, it should be taken into account that the pain has a sensory and an emotional component that condition all the characteristics inherent to pain in itself and individually in each person. According to the IASP, the sensory component, that is, that perceived by the peripheral nociceptors, has the same importance as the emotions, the particular context and the feelings involved with the painful experience. Taking into account the results obtained by Mameli, Yang, and Singer et al., among others, patients experience pain improvement significantly superior to that obtained with placebo as a result of the appearance of positive emotions, improvement of affect and emotional reconstitution. activation of the OXT system (17,18). OXT stimulates receptors at pre and postsynaptic level, initiating a succession of events that end in the release of endogenous opioids. The opioid system produces a phenomenon of central modulation inducing synaptic rearrangement, and plastic changes that modify the experience and positively affect the tolerance of the patient in the presence of a potentially nociceptive stimulus (5). Consequently, a significant change in the emotional condition of the patient and his affection, a significant improvement in the painful experience and in the individual report of pain. It can be assumed that OXT, in various presentations, modifies the nociceptive experience, and in spite of not treating the pathophysiological cause of chronic pain, it makes the patient have less pain. In fact, the results obtained by Mameli et al. indicate that depression decreased in both the control and experimental groups, from the baseline to the third week (47.2 with SD 12.1 to 45.9 with SD 12.0 in the control group and went from 48.2 with SD 12.2 to 46.2 with an SD 12.6) (19).
It can be argued that the evidence provided by most studies is related to the activation of the OXTnergic system.
Based on the review carried out, we consider that OXT has a great potential as an adjuvant in adult patients with chronic pain and that it is necessary to rigorously evaluate its safety profile and its adverse effects.
INTEREST CONFLICT
The authors declare that there is no conflict of interest.
REFERENCES