
DOI: 10.20986/resed.2020.3796/2020
ORIGINAL
Efficacy of lidocaine infusions in fibromyalgia
Eficacia de las perfusiones de lidocaína en la fibromialgia
M. Verd, Hermann Ribera, C. Sansaloni, M. J. de Vicente and M. Truyols
Unidad del Dolor, Servicio de Anestesia, Reanimación y Terapéutica del Dolor, Hospital Universitario Son Espases. Palma de Mallorca, España
Verd M, Ribera H, Sansaloni C, de Vicente MJ, Truyols M. Efficacy of lidocaine infusions in fibromyalgia. Rev Soc Esp Dolor. 2020;27(5):287-291
Received: 15-02-2020
Accepted: 07-09-2020
Correspondence: Mateo Verd
mverdster@gmail.com
ABSTRACT
Introduction: Fibromyalgia is defined as a pain syndrome characterized by generalized chronic pain, fatigue, sleep disturbances, anxiety and depression and, at times, an insufficient and frustrating treatment
response. It is a benign condition chronically suffered by
many patients, which forces the medical community on
an ongoing search for an optimal treatment. We evaluated
the effect of intravenous lidocaine on the different
characteristics of fibromyalgia.
Method: This is a prospective, longitudinal study. We
recorded data for 48 patients diagnosed with fibromyalgia
syndrome according with the American College of
Rheumatology criteria (ACR 1990). Patients with abnormal
electrocardiogram or abnormal blood electrolytes
were excluded. Included cases received an increasing
dose of 2 mg/kg up until 5 mg/kg of an intravenous lidocaine
perfusion during 10 days. All included cases filled
the following questionnaires at day 0 (pre-treatment), day
10 (post-treatment) day 30 and day 90: Health Survey
SF-12 (SF-12), Fibromyalgia Impact Questionnaire (FIQ),
Brief Pain Inventory (BPI), Big Five Inventory (BFI), Beck
Depression Inventory II (BDI-II), Medical Outcomes Sleep
Scale (MOS), Patient Improvement Expectations (EXPEC).
Results: 48 cases were recorded, 46 were female
and 2 were male, the median age was 55 (36-70). We
found improvement in pain, fatigue and psychological
attitude at 10 days that disappears at 30 days.
Discussion: The treatment with infusion of intravenous
lidocaine at these doses on this set of patients did
not modify the clinical characteristics of fibromyalgia as
a sustained manner.
Key words: Lidocaine, fibromyalgia, central sensitization, chronic pain, neuropathic pain.
RESUMEN
Introducción: La fibromialgia se define como un síndrome de dolor crónico benigno caracterizado por dolor generalizado, fatiga, alteración del sueño, ansiedad y depresión, y en muchas ocasiones una respuesta al tratamiento insuficiente y frustrante que obliga a la comunidad médica a un esfuerzo continuo en la búsqueda de un tratamiento óptimo. Hemos evaluado el efecto de la lidocaína intravenosa en perfusión sobre las diferentes características de la fibromialgia.
Métodos: Después de la aprobación del comité de ética de nuestro hospital y el consentimiento informado de cada paciente, se incluyeron 62 pacientes con criterio de fibromialgia según la American College of Rheumatology. Se realizaron perfusión de lidocaína a dosis creciente desde 2 mg/kg hasta 5 mg/kg durante 10 días. En todos los casos se rellenaron los siguientes cuestionarios en el día 0 (pretratamiento), día 10 (postratamiento) día 30 y día 90: Health Survey SF-12 (SF-12), Fibromyalgia Impact Questionnaire (FIQ), Brief Pain Inventory (BPI), Big Five Inventory (BFI), Beck Depression Inventory II (BDI-II), Medical Outcomes Sleep Scale (MOS), Patient Improvement Expectations (EXPEC).
Resultados: De los 62 pacientes, 9 abandonaron el estudio por efectos adversos intolerables y 5 se perdieron o no completaron los cuestionarios en los siguientes meses. Finalmente completaron el estudio 48 pacientes, 46 mujeres y 2 hombres con una edad media de 55 (36-70) años. Encontramos una mejoría en los cuestionarios BPI, BFI y BDI-II en el día 10 que no se mantienen a los 30 días.
Discusión: En nuestro estudio el tratamiento con perfusiones de lidocaína en pacientes con fibromialgia objetivó una buena eficacia inicial que no se mantuvo con el paso del tiempo.
Palabras clave: Lidocaína, fibromialgia, sensibilización central, dolor crónico, dolor neuropático.
Verd M, Ribera H, Sansaloni C, de Vicente MJ, Truyols M. Efficacy of lidocaine infusions in fibromyalgia. Rev Soc Esp Dolor. 2020;27(5):287-291
Received: 15-02-2020
Accepted: 07-09-2020
Correspondence: Mateo Verd
mverdster@gmail.com
INTRODUCTION
Fibromyalgia is a medical condition characterized by chronic widespread pain, associated with other symptoms, such as fatigue, insomnia, memory loss, mood swings, tremors, paresthesia, cramps, morning stiffness, and multifocal digital pressure hyperalgesia on physical examination. The pathophysiological substrate is the sensitization of the central nervous system, so pain perception is amplified from the peripheral pain signal that is transmitted through the spinal cord to the brain. There is no complementary examination capable of diagnosing this benign disease and the diagnosis is primarily clinical.
There is no known cure to date, although a palliative treatment of symptoms is available, so optimal treatment of fibromyalgia includes pharmacotherapy despite the limited scientific evidence about its efficacy (1). Clinical experience corroborates this fact; pain relief while maintaining good tolerance for adverse effects is difficult to achieve in these patients, so there is a large group of patients who are refractory to oral analgesic treatments. This type of patients may be candidates for alternative analgesic treatments in the form of continuous intravenous infusion in an attempt to relieve their pain consistently over time.
Lidocaine was first synthesized in 1942 (initially called xylocaine) and marketed in 1948, and the first publications date from the decades of the 1950s and the 1960s (2,3). In addition to its use as a local anesthetic and anti-arrhythmic drug, its analgesic properties were soon documented in various painful conditions. Intravenous lidocaine infusions have been shown to have an antihyperalgesic effect in chronic central and peripheral neuropathic pain conditions in short-term studies conducted with few patients (4-6). In fibromyalgia, there are few studies on the efficacy of continuous infusions of lidocaine and, moreover, few studies report the duration of its analgesic effect and whether its benefit is consistent enough to maintain it as an appropriate treatment for these patients (7). There is evidence that lidocaine acts mainly by blocking the sodium channels by binding in segment 6 of domain 4 of the alpha subunit of the ion channel and inhibiting neuronal ectopic discharges, although the exact molecular mechanisms of action are not fully known, probably because preclinical studies suggest that it may be due to their cellular, subcellular, regional, and systemic actions that ultimately shape its analgesic properties (8).
The primary objective of this study is to evaluate the efficacy of intravenous lidocaine infusions in our fibromyalgia patients, as well as their impact on the quality of life of these patients during the next three months after treatment.
METHODOLOGY
The study was approved by the Ethics Committee of our hospital, and an informed consent was signed by the patients after obtaining verbal and written information. The clinical trial met the requirements of good clinical practice and established quality standards.
Patient selection
Patients were selected from January to December, 2017. The inclusion criteria were: At least 18 years of age and suffering from chronic widespread pain for at least three months under the diagnosis of fibromyalgia according to the American College of Rheumatology criteria (9). Patients with a previous history of arrhythmia or heart disease, abnormal electrocardiogram, and with a blood electrolyte imbalance (sodium, potassium, magnesium, phosphorus, and calcium) were excluded. Patients were instructed to continue to take their usual chronic medication during treatment until the end of treatment.
Intervention
After the cannulation of a peripheral vein, each patient received intravenous lidocaine infusion daily for 10 consecutive days at increasing doses and for 2 hours: 2 mg/kg on day 1, 3 mg/kg on day 2, 4 mg/kg on day 3 and 5 mg/kg on the remaining days if tolerance to adverse effects allowed it, otherwise the minimum well-tolerated dose for each patient was maintained until the end of treatment. We have chosen these doses based on previous studies starting with a minimum dose and titrating to avoid the occurrence of adverse effects (10-13). During treatment, the patient was monitored hemodynamically and breathlessly with continuous electrocardiogram monitor, pulse oximetry, blood pressure every 10 minutes and under surveillance by the nursing staff. Patients were asked to notify any symptoms consistent with any adverse treatment effects during treatment delivery.
Outcome measures and follow-up
Patients completed the following questionnaires: the SF-12 Health Questionnaire, the Fibromyalgia Impact Questionnaire (FIQ), the Brief Pain Inventory (BPI), the Big Five Inventory (BFI), the Beck Depression Inventory-II (BDI-II), the Medical Outcomes Study (MOS) Sleep scale, and the patient’s expectations for improvement (EXPEC). These questionnaires were completed at the following times: before starting the first treatment (baseline or day 1), immediately after the last treatment (day 10), at 1 month (day 30) and at 3 months (day 90).
Statistical analysis
For each outcome measure, the change from baseline to any of the follow-up days was assessed using the Wilcoxon p-test. Unless otherwise noted, the data are expressed as median and range.
RESULTS
A total of 62 patients were included in the study, 9 of them withdrew due to the appearance of intolerable lidocaine adverse effects, 8 patients due to nausea and 1 patient due to worsening of the pain, that forced discontinuation of treatment, whereas 5 patients were lost to follow-up or failed to respond to questionnaires within the following months. Finally, 48 patients completed the study. Table I shows baseline data of the patients.
TABLE I. DEMOGRAPHIC DATA

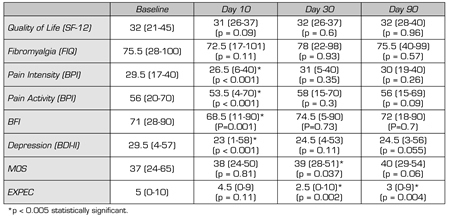
Table II shows the results of the questionnaires. Statistically significant differences were found between baseline and day 10 (end of treatment) in the BPI questionnaire, in both pain intensity (29.5 to 26.5; p < 0.001) and pain activity (56 to 53.5; p < 0.0019). Statistically significant differences were also found in the BFI questionnaire (71 to 68.5 p = 0.001) and in the BDI-II questionnaire (29.5 to 23; p < 0.01). However, these differences are not maintained within the following days after treatment except for the MOS questionnaire at day 30 (from 37 to 39; p = 0.037) and for patient expectations (from 5 to 2.5; p = 0.002). At three months, no differences in questionnaire results were found compared with the baseline situation.
TABLE II. QUESTIONNAIRES OUTCOME MEASURES ON DAYS 1, 10, 30 AND 90
DISCUSSION
In our study, treatment with lidocaine infusions in patients with fibromyalgia showed a good initial efficacy in terms of decreased pain intensity, improved functional capacity and mood. However, this improvement at the end of treatment did not show consistency over time. Sleep quality and patient expectations only improved at the first month post-treatment, whereas no difference was found in the analyzed questionnaires at three months post-treatment compared with the baseline.
Similarly to previous studies, the majority of patients were women (10,14) and the age of patients was similar to those reported in the literature, with a higher prevalence between 40 and 60 years old (15).
The doses of lidocaine infusions received by the patient based on our protocol established a progressive increase of the infusions according to the patient’s tolerance to the medication administered. That is, the optimal titration of the administered dose was individualized and limited by the appearance of adverse effects, most of them were mild, such as metallic taste, dizziness, tremors and palpitations, or nausea and vomiting as more frequent, and it meant stabilization of optimal doses between 2 and 5 mg/kg/h until treatment completion in a two-hour infusion (i.e. total dose ranged from 1 to 10 mg/kg). The doses used by several researchers range from 1 to 5 mg/kg administered over an approximate period of 30-60 minutes (11,16). However, a recent study reports that doses of 7.5 mg/kg are more effective than 5 mg/kg in terms of pain relief and duration of its analgesic effect (17).
Previous studies have shown the efficacy of lidocaine infusion in various pain syndromes including fibromyalgia. The prolonged efficacy of its analgesic effect has been shown in two case series published in 1995 for more than 30 days (12) and another in 2015 for more than 5 weeks (18). In a retrospective study conducted with 50 patients, significant pain relief was reported for 11.5 ± 6.5 days after six days of intravenous lidocaine infusions (13). An open-label study found decreases in the scores of the Visual Analog Scale (VAS) and the FIQ immediately after the fifth day of perfusion and 30 days after the fifth infusion (19). In two randomized, controlled studies published in 1995 and 2000, a long-term pain relief was evidenced by decreased VAS score for more than 4 weeks (20,21).
Therefore, the analgesic effect of lidocaine infusions appears to be longer than its pharmacokinetic half-life of approximately 120 minutes and its metabolites half-life (more than 12 hours) (22), probably due to the decrease of spinal sensitization as suggested by some authors (10).
Finally, intravenous lidocaine is associated with dose-dependent adverse effects including dizziness, sedation or tinnitus, and at higher doses it is associated with seizures and arrhythmia. No severe adverse effects were found in our study. In a randomized study with intravenous lidocaine infusions, the most common adverse effects were metallic taste, tremor, dry mouth, insomnia, allergic reactions, and tachycardia, and no severe adverse effects, such as heart arrhythmias or hemodynamic instability, were described (23).
Our study has limitations due to the lack of a placebo-matched control group and it should have been randomized and double-blind. In addition, we suggest that other outcome variables should be included in further studies, such as daily pain relief, adverse effects during and after infusions, analgesic use, and concurrent use of medication after lidocaine infusions.
CONCLUSIONS
In our study in diagnosed patients, intravenous lidocaine infusions at optimal doses titrated according to individual tolerance between 2 mg/kg and 10 mg/kg administered for two hours showed efficacy immediately after 10 days. However, the questionnaire scores showed that the effect of these lidocaine infusions was not prolonged in time neither in the first nor in the third month after treatment. Currently in our usual practice we have discontinued the usual use of this therapy given the discouraging results and we reserve this treatment for those selected cases that report finding sustained relief with this therapy. The decision to use it in new cases is more complicated and it is left at the discretion of the physician in charge of the patient, but in general, intravenous lidocaine infusions are reserved for patients with poor quality of life not responding to other therapies.
CONFLICT OF INTEREST
The authors of the present study have no conflict of interest to declare.
REFERENCES