DOI: 10.20986/resed.2019.3724/2019
REVIEW
Orofacial pain in the dental clinic
1Área de Estomatología. Dpto. Medicina y Cirugía, Psicología, Medicina Preventiva y Salud Pública, Inmunología y Microbiología Médica, Enfermería y Estomatología. Facultad de Ciencias de la Salud. Universidad Rey Juan Carlos (URJC). Alcorcón, Madrid. 2Área de Farmacología, Nutrición y Bromatología. Unidad asociada I+D+i al Instituto de Química Médica (CSIC). Grupo de Excelencia investigadora URJC-Banco de Santander-Grupo Multidisciplinar de Investigación y Tratamiento del Dolor (i+DOL). Dpto. Ciencias Básicas de la Salud. Facultad de Ciencias de la Salud. Universidad Rey Juan Carlos (URJC). Alcorcón, Madrid. España
Received: 16-02-2019
Accepted: 23-04-2019
Correspondence: Blanca del Carmen Migueláñez Medrán
blancac.miguelanez@urjc.es
ABSTRACT
Most dental consultations are related to intraoral pain disorders affecting dental, periodontal and mucosal structures. Although the originating cause of pain and the anatomical structure frequently co-localise, orofacial pain and particularly oral pain are sometimes referred. That is, pain may be caused by extraoral processes out of the maxillofacial territory. Likely, some intraoral conditions such as an occlusal imbalance may also affect extraoral structures, leading to tension and pain on the neck, head, and back. Orofacial pain research is however an emerging discipline in comparison to other anatomical regions. This may be due, in part, to the fact that oral pain tends to recede over time or after tissue healing –in case there was an injury–. Notwithstanding, half of the patients reporting any sort of orofacial pain suffers chronically. And unlike acute receding pain, chronic pain is no longer a symptom, but a difficult-to-manage pathology, with scarce or none relation to the mechanisms that caused it. Moreover, the lack of appropriate anamnesis and clinical examinations, inaccurate pain syndrome nomenclatures or difficulty in diagnosis hamper sometimes an optimal therapeutic approach. Most oral pain classifications are still based on the affected anatomical structure rather than on the nociceptive mechanism itself. On the other hand, the precise aetiology of most of the so-called atypical algiae or the burning mouth syndrome is still unknown. The present review article aims to describe the main reasons for pain consultation at the dental clinic, with particular emphasis on the type of pain from a mechanistically point of view: nociceptive, inflammatory, neuropathic, psychogenic or mixed.
Key words: Orofacial pain, neuralgia, odontalgia, oral cancer, temporomandibular joint pain.
RESUMEN
La mayor parte de las consultas odontológicas están relacionadas con dolores intraorales que afectan a estructuras dentarias, periodontales y mucosas. Aunque generalmente la causa originaria del dolor y la estructura afectada coinciden en la localización, en ocasiones el dolor orofacial y, particularmente, el dolor oral, es referido. Esto es, el dolor puede deberse a procesos de origen extraoral localizados fuera del territorio maxilofacial. De igual manera, determinados trastornos orales, como un desequilibrio oclusivo, pueden afectar también estructuras extraorales, ocasionando tensión y dolor en cuello, cabeza y espalda. La investigación en dolor orofacial es, sin embargo, una disciplina emergente en comparación con otras áreas anatómicas, quizás debido, en parte, a que el dolor tiende a remitir con el tiempo o con la sanación del tejido afectado (si hubiera una lesión). Sin embargo, la mitad de los pacientes con algún tipo de dolor orofacial lo sufre de manera crónica y, a diferencia del dolor agudo, remitente, el dolor crónico no es ya un síntoma, sino una patología de difícil manejo, con escasa o ninguna relación con los mecanismos que lo originaron. Además, la falta de una adecuada anamnesis y exploración clínica, nomenclaturas inapropiadas o la dificultad de diagnóstico, hacen complicado en ocasiones un óptimo abordaje terapéutico. La mayoría de las clasificaciones de dolor oral siguen atendiendo a la estructura anatómica afectada más que al propio mecanismo nociceptivo. Por otra parte, la etiología exacta de muchas algias denominadas atípicas o del síndrome de boca ardiente sigue siendo desconocida. Esta revisión pretende describir los principales motivos de consulta por dolor en la clínica dental, poniendo particular énfasis en el tipo de dolor desde el punto de vista de su mecanismo: nociceptivo, inflamatorio, neuropático, psicogénico o mixto.
Palabras clave: Dolor orofacial, neuralgia, odontalgia, cáncer oral, dolor articular temporomandibular.
INTRODUCTION
Most patients attending the dental clinic complain of odontalgia which, in general, is of an acute nature. However, the pain of the temporomandibular and myofascial muscles, in conjunction with neuralgia, are among the different types of chronic pain with a higher incidence in the dental practice (1-3). Headaches are another group of great frequency, but in the general population (4,5). In fact, according to the Spanish Pain Society (SED, from Spanish Sociedad Española del Dolor), half of the patients with orofacial pain in the general population (that is, without considering exclusively those patients who visit the dentist) suffers this pain chronically. All these types of pain are complex to treat, being more common among women (with the exception of dental pain) (6,7) and decreasing their prevalence usually with age (8).
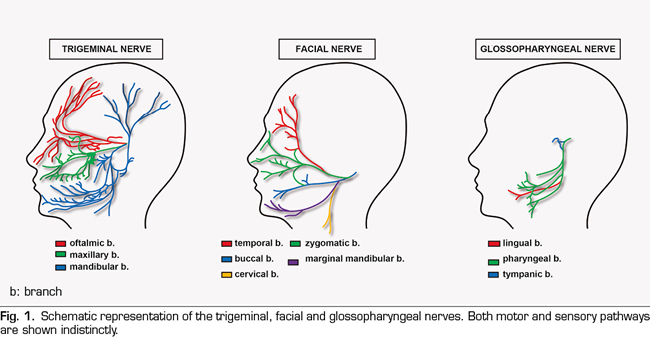
Given the abundant and intricate innervation of the regions associated with orofacial pain (Figure 1), it is not surprising that sometimes it is so difficult to categorize (9), which may condition its therapeutic approach and, consequently, the efficacy of the treatment. In addition, the existence sometimes of a pain with a strong psychogenic component, (2) and even of referred type (10), makes even more difficult (if possible) to find an effective diagnosis and treatment. It is precisely the existence of cranial nerves, beyond the spinal cord, which demands and justifies a close collaboration between experts in the stomatognathic system, psychologists and various specialist doctors, beyond that existing in the Pain Units for other anatomical regions. That is, the development of orofacial pain units is necessary (11,12).
Moreover, the limited knowledge about certain types of orofacial pain pathologies by various healthcare professionals is not a rare occurrence, as it is reflected in monographs published by the Spanish Association of Dentists and Stomatologists (13). Therefore, the present review aims to describe and classify the main reasons for consultation that the dentist may find in daily practice, with the appearance of a painful process as a trigger for the dental visit as a common denominator.

MAIN TYPES OF OROFACIAL PAIN
Most patients who experience some type of orofacial pain attend to their family doctor or dentist and are usually treated by them. However, sometimes the patient must be referred to a specialist doctor or even to a pain unit.
In line with the above, although a high percentage of pain has its origin in dental, periodontal and mucosal structures, there are certain conditions that can find painful processes in these same structures derived from other extraoral locations (10). One of the characteristics to take into account in the diagnosis of orofacial pain (and more specifically in oral pain) is the fact that pain can have a diverse origin (dental, oral or even systemic), also influenced by other subjective sensations of the patient, such as depressive behavior or anxiety (14). Reaching a correct diagnosis is sometimes difficult, because many types of pain, even with different mechanisms of nociception, share the same signs and symptoms (15,16).
Odontalgias
The dental pulp is densely innervated by polymodal C nociceptors, but also by Aδ and Aß fibers, which makes possible to respond to stimuli of different origin. It is thought that, in the majority of cases, the dental pain is the consequence of an inflammatory process of the pulp and its duration and intensity generally depends on the magnitude of the damage, decreasing when the stimulus producing it is reduced. The main reason for tooth hypersensitivity is, therefore, the exposure of the dentinal tubules to thermal or mechanical stimuli, to the intake of sweet foods or to the pressure exerted by brushing (17). The triggering cause is usually the exposure of the dentin to processes of attrition, abrasion or erosion (such as that produced by caries)[18,19]), although it may also be due to the exposure of the root surface of the tooth secondary to a periodontal disease or derived from a surgical intervention (20). In contrast, the existence of algoneurons has been proposed: low threshold mechanical Aβ fibers that would transmit nociceptive signals in the absence of inflammation or central sensitization when mechanically stimulated (for example, by a breath of air or a jet of water); that is, they would be constitutively active in the healthy tooth and would be exposed when enamel and dentin are eroded (21).
The fracture of a tooth can also lead to a painful process known as cracked tooth syndrome. The difficulty in managing this type of pain is that the detection of the fracture and its depth are difficult to assess because there is no structural loss or visible separation of the tooth structure (22). The methods used for its visualization in the dental clinic consist of transillumination, radiographic techniques or the application of methylene blue (23,24). However, it is not always possible to see such a fracture (25), which hinders diagnosis and treatment. Its treatment depends on the depth of the crack and the affected tissue: enamel, dentin, pulp cavity and/or root surface (22).
Sometimes, infections or lesions of the posterior teeth, improperly performed sinus elevations, root canal overfilling in endodontics, complications arising from the placement of dental implants and even oro-antral fistulas resulting from a tooth extraction can damage the oro-antral membrane, resulting in a maxillary sinusitis of odontogenic origin (26-30). The main consequence is a perception of bad smell by about half of the patients themselves and an increase in susceptibility to microbial infections. Although previous studies claim that only ~30% of these patients have dental pain (31), they are not exempt from treatment or extraction of the affected piece, antibiotic treatment, as well as being referred to the maxillofacial surgery service (29,32).
Superficial somatic pain: mucosa and periodontium
Recurrent aphthous stomatitis is the most common disease of the oral mucosa. It occurs with recurrent ulcerations that cause pain, persisting for days or weeks (33,34). Its etiology is still unknown and the current treatment is symptomatic and aimed at reducing the number and size of ulcerations (35). There is also a group of autoimmune diseases affecting the skin and oral mucosa, accompanied by inflammatory-type pain. Two of these diseases are pemphigus, in which blisters occur on the skin surface and mucosa, and pemphigoid, affecting almost exclusively mucous membranes, which can affect from the oral to the nasal, ocular or even genital mucosa (36). Oral lichen planus is another disease of a particular autoimmune nature. It appears in the form of white lesions of the mucosa that produce pain, burning and stinging (37). In both cases, the treatment consists of the local application of corticosteroids; the use of retinoids, immunomodulators or phototherapy can be used in more severe cases for skin lesions, as well as the use of antiseptics and analgesics to control pain. The main complications in the treatment of this type of pathologies lie in their recurrent nature and in the potential risk of developing mycosis when patients undergo long-term treatment with corticosteroids (38).
There are also different types of periodontal pain. One of the main causes of pain of periodontal origin is due to occlusal trauma, in which the occlusal forces on the periodontium exceed the adaptive capacity of the tissues. If this increase in occlusal forces is maintained over time, it can also lead to joint-type pathology. Its treatment includes analgesics and the performance of occlusal adjustments to reduce the force applied to the affected dental piece, correction and managing of parafunctional habits, splinting of the pieces with mobility, orthodontic treatment, occlusal reconstruction with different prosthetic treatments or ultimately extraction of the piece involved (39). Two other specific forms of periodontal disease are necrotizing ulcerative gingivitis and necrotizing ulcerative periodontitis. Both are characterized by an acute process of severe gingival pain, papillary necrosis and bleeding (40,41), with the difference that the latter involve also bones (42). In addition to symptomatic treatment with analgesics, the pharmacological therapy includes the use of antibiotic therapy combining amoxicillin and metronidazole and antibacterial (chlorhexidine) or antiseptic (hydrogen peroxide) rinses. It is also necessary to use mechanical therapy to eliminate bacterial plaque (43).
Various conditions of the salivary glands can also involve pain. Necrotizing sialometaplasia is an inflammatory process generating an ulcerated surface, painful or not, in the salivary glands of the hard palate (44-46). Its appearance is mainly associated with the application of anesthesia on the hard palate and with the vasoconstrictor effect that anesthesia produces (47). In contrast, although it also has an inflammatory nature, acute necrotizing sialadenitis is a process of unknown etiology affecting mainly the minor salivary glands and it is characterized by the appearance of a severe pain in the hard or soft palate or tonsils of an approximate duration of 5-6 weeks. Another condition of the salivary glands is sialolithiasis, which occurs with the formation of salivary stones (sialoliths) in the parenchyma of the duct of a salivary gland (48). The occlusion of the duct prevents the passage of saliva and there is an increase in intraductal pressure, responsible for the appearance of painful sensation and swelling (49). However, in some cases pain is not experienced (50). In this case, foods or even drugs (sialogogues) can be administered to stimulate the salivary secretion and, in this way, lead to the expulsion of the sialolith. In addition, the treatment may require antibiotic therapy to avoid an infectious process, drainage of the gland, removal of the sialolith (51) or even fragmentation by ultrasound (52).
Various bacterial, mycotic and viral infections of the oral cavity can present with pain, being the treatment for all of them exclusively symptomatic, with analgesics and antibiotics, antifungals or antivirals. Both acute and chronic inflammations can affect the major salivary glands (parotid, sublingual and submandibular) and, to a lesser extent, the minor glands (53). The parotid gland is the most affected by these infectious processes (54,55). For this purpose, acute bacterial parotitis produces an inflammatory swelling characterized by the appearance of severe pain, fever and malaise. The same symptoms occur in acute epidemic parotitis (mumps) of viral origin (56). In addition, the contagion of the herpes simplex virus is produced by contact of a healthy individual with an infected individual with active skin or oral mucosal lesions or by fomites. Once the herpetic primary infection is produced, the virus remains dormant and its reactivation may lead to herpes labialis or intraoral herpes, with the appearance of multiple vesicles that will join forming larger ulcers. These ulcerations are characterized by burning, tingling and painful sensations (57). Among fungal infections, candidiasis is the most common in the oral cavity. Oral candidiasis presents with white or erythematous lesions located on the tongue, buccal mucosa, palate, alveolar ridges, tonsils and even esophagus (58,59) and, sometimes, these lesions can cause odynophagia and dysphagia.
Despite the recurrent nature of most of these conditions, all of them involve a type of acute pain that is punctual, solvable, or at least capable of being reduced to a great extent with the commonly prescribed analgesics.
Burning mouth syndrome
The burning mouth syndrome constitutes a separate chapter within the different types of orofacial pain. Its main characteristic is the appearance of a painful sensation of burning or stinging in the anterior part of the tongue, although other locations such as the palate, alveolar ridges, buccal mucosa and lips may be affected (60). It has a duration of, at least, 4 or 6 months, without being able to show physical injuries, that is, the oral cavity presents an aspect without any objective pathology at the clinical examination. It mainly affects women of postmenopausal age (61) and its prevalence has been estimated at 0.7-4.6% in the general population (62). It is a chronic disease and, although the implication of neuropathic and psychogenic components is suspected, the therapy used remains poorly effective and complicated to manage (63-65). Currently, the treatment consists of a multidisciplinary approach, with the topical application of capsaicin, benzodiazepines and corticosteroids, as well as the use of psychological therapy (66).
Deep somatic pain: muscle and joint pain
The functional conditions of the stomatognathic system affect around 80% of the world population (67) and, for this purpose, temporomandibular disorders are one of the most frequent causes of chronic orofacial pain (68), affecting both the temporomandibular joint and the masticatory muscles and adjacent structures (69). Another of the most frequent conditions in the general population, and therefore of visits to primary care centers (70,71), it is represented by myofascial pain. Although it is characterized by the appearance of trigger points on facial muscles, its etiology is still not completely known and, in the absence of a specific treatment (72), currently, a multi-therapeutic approach is chosen: manual physical therapy, electrotherapy, low intensity laser, ultrasound therapy, dry needling, non-steroidal anti-inflammatory drugs (NSAIDs), lidocaine patches, as well as muscle relaxants or benzodiazepines (71). The high presence of these types of pain in society, in addition to its difficult therapeutic management, makes necessary further study on its etiology, pathophysiology and treatment in general.
Neuropathic pain
Neuralgia is a type of orofacial pain with a prevalence that is difficult to calculate, in many cases due to its unknown etiology. Although the study of orofacial neuralgia has traditionally been limited to the trigeminal nerve, new classifications are recently being suggested for the study of different neuralgias: trigeminal neuralgia, atypical trigeminal neuropathic pain, persistent idiopathic facial pain (divided into atypical facial pain and atypical odontalgia), neuralgia of the intermediate nerve of Wrisberg (or geniculate), neuralgia of the glossopharyngeal nerve, neuralgia of the superior laryngeal nerve, postherpetic neuralgia, atypical neurovascular pain, phantom tooth pain, etc. (73,74).
Trigeminal neuralgia represents one of the most complex to treat orofacial pains. The nervous damage can present a different location and etiology, having been developed to date a dichotomous classification: classic neuralgia, produced by microvascular compression at the entrance of the nerve to the brainstem, and symptomatic neuralgia, all the others (75). The painful sensation can last from a few seconds to several minutes and, although many patients present peaks of pain, this is usually present to a greater or lesser extent in a constant manner (76). Its prevalence is not yet well known (77,78) and the pharmacological treatment corresponds mainly to the use of anticonvulsants/antiepileptic drugs, although antipsychotics and benzodiazepines are also prescribed (76). Some patients, however, seem to be refractory to pharmacological treatment, and there are currently other therapeutic options for them: surgical treatment of the Gasserian ganglion using percutaneous techniques or by microvascular decompression (79,80).
Although persistent idiopathic facial pain has traditionally been classified as a somatic pain affecting muscle structures due to its dull and hard locatable character (in contrast with trigeminal neuralgia, characterized by a sharp and severe pain) (65), the persistence of this type of pain over time and its difficult diagnosis suggest a neuropathic involvement (76,79). Although in most of the cases the dental pain of the patients attending a dental clinic corresponds to an identifiable oral process and, therefore, the treatment is prescribed according to the etiology of the process that causes the pain (81), sometimes we find atypical odontalgias that also seem to imply a mixed or neuropathic component more difficult to treat (82). This pain can originate from both the complication of the lesion of a dental piece or subsequent to its extraction (phantom tooth syndrome), but without any clinical or radiographic signs evidencing existing pathology (83). In addition to its neuropathic nature, atypical odontalgia (and orofacial neuralgia as a whole) generally involve a strong psychogenic component (84); therefore, its pharmacological treatment includes the prescription of antidepressants and/or antipsychotics, benzodiazepines or antiepileptics (85-88).
The jaw pain of cardiac origin deserves special mention. Acute myocardial ischemia usually presents with retrosternal pain that can project to the arms, neck and jaw. However, in certain cases, the pain is confined to the maxillofacial territory, often in the neck and jaw, although we also find it in the form of intraoral pain. The latter could be explained by the interneuronal connections between the medullary levels of the trigeminal nerve and the upper cervical roots (10).
Cancer pain
Squamous cell carcinoma (or epidermoid carcinoma) is the most frequent malignant tumor in the oral cavity (~ 90% of malignant tumors found in the oral region) (89,90). It is characterized by an invasive growth, a very high rate of early recurrences and frequent metastases in the cervical lymph nodes (91). It sometimes presents with pain in advanced stages, being asymptomatic in early stages, inflammation and changes in the oral mucosa (92). Because patients with an advanced stage of oral carcinoma have a poor medium to long-term prognosis (93), early diagnosis and recognition of certain precancerous lesions are of vital importance (94).
Additionally, the radio/chemotherapy treatment in cancer patients is not free of oral complications, regardless of the location of the tumor. In this regard, mucositis is a consequence of oncological treatment that appears in intraoral sites covered by non-keratinized mucosa (labial and buccal mucosa, ventral and lateral surfaces of the tongue, soft palate and floor of the mouth) (95). In addition to pain, oral mucositis generates a higher probability of infections, hinders the intake and, therefore, the rate of comorbidity in this type of patients is high (96). At present, the treatment is eminently symptomatic, concomitant to oncological treatment.
Headaches
The management of headaches experienced an evident improvement with the creation in 1988 of the International Headache Classification, with which, not only the diagnosis, but also the knowledge of the frequencies of the different types of pain in the society and their respective treatments have evolved widely (97). Although the pathophysiological basis of the different types of headaches can be very diverse, in all of them there is a sensitization of the afferents of the trigeminal nerve (intra or extracranial) (98).
Among the different headache types, tension headache is the most common headache (97), with a prevalence of around 40% (99). However, its mild-moderate intensity and its difficult diagnosis have favored being undertreated compared to other types of headaches with more severe and localized pain. In fact, today the treatment of tension headache is not usually pharmacological, but rather responds preferentially to physiotherapy techniques. Although the specific pathophysiological mechanism is not known with accuracy, an increase in the sensitivity and hardness of the pericranial myofascial tissues seems to precede this type of pain (100), being the participation of psychogenic factors also suggested as another possible cause, although it is still to be determined whether in an alternative or complementary manner. Moreover, recurrent headache has been identified as a neurological disorder also of high prevalence in the general population (101).
In certain cases, there are factors that predict the appearance of headache, as in the case of migraines or cluster headache, which may be preceded by a previous migraine aura. The treatment of these types of pain depends to a large extent on the etiological triggering agent. In most cases, non-steroidal anti-inflammatory drugs (NSAIDs) or opioids are chosen. However, perhaps due to unawareness of the pathophysiology of these processes, pain relief cannot be considered adequate in many cases (102).
Other studies have suggested that the combined administration of acetylsalicylic acid, paracetamol and caffeine is more effective than the consumption of each component in isolation or even more effective than the combination of only two of them (103). In contrast, the efficacy of verapamil and divalproex sodium have been demonstrated in headaches after the removal of a cranial tumor (104). However, the lack of appropriate animal models for the study of these types of pain could explain in part the scarce knowledge about their pathophysiology and the refractory or ineffective treatment in many of these patients.
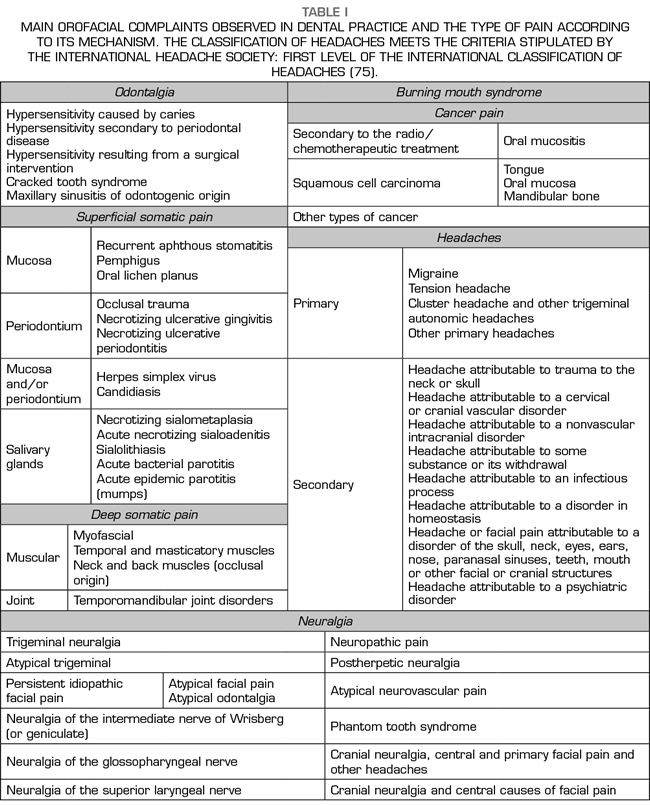
In summary, the main orofacial complaints observed in dental practice and the type of pain according purely to the mechanism of action are shown in Table I.
CONCLUSIONS
Despite the existence of classifications for different types of orofacial pain, reviews based on clinical evidence make visible the lack of a common nomenclature and methodology. This complicates not only the diagnosis, but also the study and therapeutic approach of the different types of orofacial pain. Studies based on surveys or clinical records often deal exclusively with descriptive terms of the sensation experienced (for example, burning, lancinating, irruptive or throbbing), actions (for example, chewing, eating or opening the mouth) or anatomical locations (sometimes in a very general way [for example, ears, around the eye, head or other regions]), without considering the type of pain according to its mechanism.
In clinical practice, the use of many analgesic drugs is conditioned by the duration and intensity of pain (thus it was considered 30 years ago in the analgesic ladder of the WHO for the management of oncological pain and it has been applied to any type of pain), when it should depend on the physiopathological mechanism proper of the type of pain. In fact, opioids, often prescribed to treat moderate-severe pain, fail to treat some types of chronic pain.
CONFLICTS OF INTEREST
Authors declare no conflicts of interest.
BIBLIOGRAPHY