DOI: 10.20986/resed.2021.3903/2021
REVIEW
A NEW PARADIGM FOR THE TREATMENT OF KNEE OSTEOARTHRITIS: THE ROLE OF HYALURONIC ACID, PLATELET-RICH PLASMA (PRP) AND OZONE IN THE MODULATION OF INFLAMMATION: A REVIEW
UN NUEVO PARADIGMA PARA EL TRATAMIENTO DE LA OSTEOARTRITIS DE RODILLA: EL PAPEL DEL ÁCIDO HIALURÓNICO, EL PLASMA RICO EN PLAQUETAS (PRP) Y EL OZONO DE LA MODULACIÓN DE LA INFLAMACIÓN: UNA REVISIÓN
M. E. Fernández Cuadros1
O. S. Pérez Moro1
M. J. Albaladejo Florín1
S. Álava Rabasa1
M. J. López Muñoz2
J. Rodríguez de Cía3
1Servicio de Medicina Física y Rehabilitación. Hospital Universitario Santa Cristina. Madrid, España
2Servicio de Farmacia Hospitalaria. Hospital Universitario Santa Cristina. Madrid, España
3Servicio de Laboratorio Clínico. Hospital Universitario Santa Cristina. Madrid, España
ABSTRACT
Introduction: Osteoarthritis (OA) is the most common cause of arthritis. Traditionally, OA was viewed as a “wear and tear” disease. However, metabolic and inflammatory factors are now being considered as pathogenic factors to the point that some authors are redefining OA as a “chronic low-grade inflammation” disease.
Evidence: In knee osteoarthritis, many inflammatory signaling pathways and mediators are involved. The new treatment paradigm is based on cellular treatments on the signaling pathways of inflammation, based on cellular and protein components to combat the inflammatory environment of the arthritic joint and regenerate damaged tissue.
Results: The approach of treating only one therapeutic target (nitric oxide inhibitors, nutraceuticals, urate reducing agents, and biologics) that have demonstrated their efficacy in the treatment of inflammatory diseases such as rheumatoid arthritis has not been translated into effective management in OA. A treatment approach aimed simultaneously at multiple targets would be able to manage OA more efficiently. The standard guidelines (AAOS, OARSI, ACR, NICE, or EULAR) do not consider hyaluronic acid, platelet-rich plasma, or ozone, although these treatment options have shown immunomodulatory and healing properties. In this scenario, we hypothesized that hyaluronic acid, platelet-rich plasma, and ozone are promising alternatives for the management of knee OA, due to their multidial properties, as will be seen in this review.
Conclusion: In the present study the pathophysiology of OA has been reviewed, focusing mainly on the inflammatory mechanism, the signaling pathways involved and the possible goals of treatment. Hyaluronic acid, platelet-rich plasma and ozone are proposed as multi-target options for the treatment of knee osteoarthritis.
Key words: Osteoarthritis, inflammation, hyaluronic acid, platelet-rich plasma, ozone, cytokines
RESUMEN
Introducción: La osteoartritis (OA) es la causa más común de la artritis. Tradicionalmente, la OA se consideraba como una enfermedad de “desgaste”. Sin embargo, los factores metabólicos e inflamatorios se están considerando ahora como los factores patogénicos hasta el punto de que algunos autores están redefiniendo la OA como una enfermedad de “inflamación crónica de bajo grado”.
Evidencia: En la artrosis de rodilla están involucradas muchas vías de señalización y mediadores inflamatorios. El nuevo paradigma de tratamiento se basa en los tratamientos celulares sobre las vías de señalización de la inflamación, basados en componentes celulares y proteicos para combatir el entorno inflamatorio de la articulación artrósica y regenerar el tejido dañado.
Resultados: El enfoque de tratar solo una diana terapéutica (inhibidores de óxido nítrico, nutracéuticos, agentes reductores de uratos y fármacos biológicos) que han demostrado su eficacia en el tratamiento de enfermedades inflamatorias como la artritis reumatoide no se ha traducido en un manejo eficaz de la OA. Un enfoque de tratamiento dirigido simultáneamente a varias dianas sería capaz de manejar la OA de manera más eficiente. Las pautas estándar (AAOS, OARSI, ACR, NICE o EULAR) no consideran el ácido hialurónico, el plasma rico en plaquetas ni el ozono, aunque estas opciones de tratamiento han mostrado propiedades inmunomoduladoras y curativas. En ese escenario, planteamos la hipótesis de que el ácido hialurónico, el plasma rico en plaquetas y el ozono son alternativas prometedoras para el manejo de la OA de rodilla, debido a sus propiedades multidiana, como se observará en esta revisión.
Conclusión: En el presente estudio se ha revisado la fisiopatología de la OA, centrándose principalmente en el mecanismo inflamatorio, las vías de señalización implicadas y los posibles objetivos del tratamiento. El ácido hialurónico, el plasma rico en plaquetas y el ozono se postulan como opciones de tratamiento multidiana para el tratamiento de la artrosis de rodilla.
Palabras clave: Artrosis, inflamación, ácido hialurónico, plasma rico en plaquetas, ozono, citoquinas
Correspondence: Marcos Edgar Fernández Cuadros
marcosefc@hotmail.com
Received: 20-02-2021
Accepted: 03-12-2021
CONTEXT
Osteoarthritis (OA) is the most common cause of arthritis. OA has a direct impact on quality of life to the extent that it is the eleventh global contributor to disability worldwide. The burden of the disease in terms of cost is such that an estimated 4 million people are affected in Spain, involving an annual cost of EUR 4378 million per year, or 0.5 % of gross domestic product (1).
OA is a major public health problem. In people over 60 years of age, 13% report symptomatic knee OA; in people over 70 years of age, 27% have radiological signs of OA; and in people over 80 years of age, 44% have radiological signs and clinical symptoms (2).
Typically, OA was considered a “wear and tear” disease. However, metabolic and inflammatory factors are new pathogenic factors. In fact, some authors are redefining OA as a “low-grade chronic inflammation” disease (3). Therefore, there is a paradigm shift for OA, from biomechanical to inflammatory theory (2,3).
Biomechanical theory states that OA is a mechanically induced disease. Joint cartilage is overloaded as a result of poor alignment, poor mechanics, and load or impact. This overload compromises the cartilage support structure, causing it to soften and degrade. Once the cartilage softens, the bone beneath it hardens and the joint breaks. If the overload is not modified, the OA will progress. Once the elasticity of the subchondral bone disappears, the progressive destruction of cartilage and bone leads to the formation of osteophytes to increase the surface area, thus decreasing the load. Finally, the progression of the joint deformity moves the weight support axis to the opposite compartment and further joint destruction continues. Total knee arthroplasty is indicated as a final treatment at this stage (4).
Inflammatory and immune theory states that in cartilage, cells are exposed to wear forces within the extracellular matrix (ECM) and on the surface of the joint. As a result, ECM is destroyed, releasing inflammatory cytokines such as IL-6, IL-8, matrix metalloproteases (MMP), leukemia inhibitory factor (LIF), and oncostatin M (OSM). These danger signals trigger second messengers, worsening the inflammatory environment. Furthermore, cartilage senescence induces apoptosis and inflammatory responses are enhanced. These cells and inflammatory signals incite an inflammatory cascade that surpasses the innate healing response, leading to a catabolic state that produces more cartilage destruction (4).
The ECM can be destroyed by inflammation or by apoptosis mechanisms, which are mediated by immune or inflammatory responses (4). In this inflammatory environment, by acting on well-known signaling cascades, cell and protein signaling could promote favorable healing responses (4). Mesenchymal stem cells reduce inflammation, fight apoptosis (cell death), self-replicate and differentiate across multiple tissues. Platelet-rich plasma (PRP) contains nearly 1200 proteins, including growth factors and anti-inflammatory cytokines (4). They block inflammation and stimulate cartilage healing (4).
The new paradigm of the management of knee OA is based on cell signaling treatments, based on cellular and protein components. Both components are expected to interact with resident stem cells, inflammatory cells, and immune cells to combat the inflammatory environment of the joint and regenerate damaged tissue (4).
Today there is no cure for OA. The goal of short-term treatment is to decrease pain and regain the quality of life and function of patients, and in the long term to slow/stop disease progression (1). New treatment options for the management of knee osteoarthritis in the form of intra-articular infiltrations include hyaluronic acid (HA), glucocorticoids, analgesics, and unproven complementary therapies, such as platelet-rich plasma (PRP) (5) or even ozone (1). However, the good results obtained with biological drugs that fight inflammation (such as in rheumatoid arthritis), based on an approach to treating only one therapeutic target, have not been found for the management of knee OA (5).
We pose that a multi-target approach would be beneficial for the treatment of knee OA because many signaling pathways and inflammatory mediators are involved in the origin of knee OA (4).
HA, PRP and ozone act on the modulation of inflammation through different mediators and signaling pathways (1,4,5), therefore these treatment options could be considered, taking into account their multitarget profile, for the management of knee OA in the near future.
The objective of the present study is to review the pathophysiology of OA, focusing mainly on the inflammatory mechanism, the signaling pathways involved and potential treatment targets, and to pose to HA, PRP and ozone as multi-target treatment options for the management of knee osteoarthritis.
STATE OF THE ART
Pathogenesis and diagnosis of osteoarthritis
With aging, the cartilage undergoes histological changes and degradation. As a result, catabolic enzymes (MMP1, MMP10, MMP13, IL-1α, IL-6, IL-7, IL-8), glycation end-products, reactive oxygen species (ROS), apoptotic and necrotic cells are released and the ECM is decomposed (6). ROS, inflammatory cytokines, and catabolic proteins are involved in the aging process of articular cartilage (6).
Acting on mesenchymal stem cells (MSC) is necessary for the repair of cartilage. Typically, some stimuli such as TGF-β (transforming growth factor β), IGF-1 (insulin-like growth factor 1), mechanical loading and hypoxia induce the differentiation of chondroblasts into specific chondrocytes and produce elastic cartilage, hyaline cartilage, and fibrocartilage (6). As a result, articular cartilage and collagen type II, IX and X are produced (6).
OA occurs because of modifiable risk factors (obesity, occupation, injury, physical activity, sports, and diet) and non-modifiable risk factors (sex, age, genetics, and hormones). OA presents as a group of signs and symptoms, including pain, swelling, joint stiffness, and muscle weakness (7).
According to EULAR (European League Against Rheumatism), at least 3 symptoms and 3 clinical and laboratory signs must be present for the diagnosis of OA. Clinical signs and symptoms include joint pain, stiffness, loss of function, crepitus, limitation of movement, and bone enlargement. Laboratory signs include ESR (erythrocyte sedimentation rate) less than 40 mm/h, rheumatoid factor less than 1:40, and synovial fluid with leukocytes less than 2000 cells/uL (8).
OA is classified in 4 degrees according to Kellgren and Lawrence (KL): In grade 1 there is a suspicion of narrowing of the joint space and possible overgrowth of osteophytes; grade 2 shows possible narrowing of the joint space and defined osteophytes; grade 3 represents definitive sclerosis and narrowing of the minimum joint space and possible bone irregularity; grade 4 is characterized by large osteophytes, significant narrowing of the minimum joint space, major sclerosis, and some bone irregularity (8).
The diagnosis of OA is clinical and radiological. Unfortunately, the initiation of OA occurs before the radiological diagnosis is made. At this stage, the x-ray is unable to detect early stages of OA. Early diagnosis will allow us to establish a preventive treatment to slow or halt cartilage destruction (9).
A biomarker is a quantifiable marker of a biological process. In OA, a biomarker should be involved in osteogenesis and inflammation. A biomarker in OA quantifies the osteogenic or inflammatory changes observed in serum, urine, or synovial fluid; and this change, either in tissue growth or degradation, could even precede radiographic changes (9).
For knee osteoarthritis, inflammatory and non-inflammatory biomarkers are considered. Non-inflammatory biomarkers include metabolic collagen biomarkers (CTX-I [C-terminal telopeptide], CTX-II), metabolic biomarkers other than collagen (proteoglycans, aggrecanases [chondroitin sulfate, keratan sulfate], non-aggrecanases [hyaluronic acid, osteocalcin, osteopontin, folastine]. Inflammatory biomarkers are divided into pro-inflammatory and anti-inflammatory. Proinflammatory biomarkers include adipokines (leptin, adiponectin, visfatin, and resistin), interleukins (IL-1β, IL-6, IL-15, IL-17, IL-18), chemokines (TNF-α), C-reactive protein (CRP), ESR and uric acid. Anti-inflammatory biomarkers include cytokines such as IL-4, IL-7, IL-8, IL-10, and IL-13 (9).
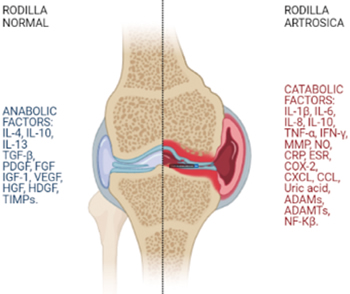
The pathophysiology of OA is very complex. In knee osteoarthritis there is an imbalance between anabolic and catabolic factors. Catabolic factors include inflammatory cytokines (IL-1β, IL-6, IL-15, IL-17, IL-18, TNF-α, LIF]) and proteolytic enzymes (MMP-1, MMP3 and aggrecanases [ADAMTs]). Anabolic factors include anti-inflammatory cytokines (IL-4, IL-10) and anabolic cytokines (TGF-β, IGF-1, FGF-18 [fibroblast growth factor] and PDGF [platelet-derived growth factor]) (10). Inflammatory and catabolic factors produce an imbalance that leads a healthy joint to develop knee OA (Figure 1) (11).
MMP: Matrix mineral metalloproteases. ADAM: disintegrin and metalloprotease. ADAMTS: disintegrin and metalloprotease with thrombospondin motifs. NO: Nitric oxide. TNF-α: Tumor necrosis factor α. iNOS: inducible nitric oxide synthase. COX-2: Cyclooxygenase-2. CXCL: Chemokine receptor. CCL: Chemokine ligand. CRP: C-reactive protein. ESR: Erythrocyte sedimentation rate. TGF-β: Transforming growth factor β. HGF: Hepatocyte growth factor. VEGF: Vascular endothelial growth factor. EGF: Endothelial growth factor. IGF-1: Insulin growth factor 1. HDGF: Hepatocyte-derived growth factor. TIMPS: tissue inhibitor of metalloproteases. NF-kβ: Nuclear factor kβ.

Fig. 1. Osteoarthritis is the result of an imbalance between anabolic and catabolic factors where pro-inflammatory cytokines and catabolic chemokines predominate over anti-inflammatory cytokines and anabolic chemokines.
Leptin is an adipokine with an inflammatory effect. An increase in body weight and an expansion of white adipose tissue lead to an increase in mechanical load; then, cartilage degradation is the end result, which triggers the development of OA. Furthermore, leptin derived from adipose tissue (overproduction in obese patients) is related to the deregulation of osteoblasts in subchondral bone tissue, leading to joint wear and tear damage. In addition, leptin releases pro-inflammatory cytokines derived from adaptive and innate immune cells, creating an inflammatory medium that favors cartilage and OA destruction (12).
In addition to leptin, other adipokines, such as visfatin, produce ROS and pro-inflammatory cytokines cause inflammation and cartilage degradation (13).
Obesity and metabolic syndrome are associated with low-grade chronic inflammation, leading to joint injury, pain, and disability. Metabolic syndrome/obesity releases IL-1β, IL-6, PGE2, TNF-α and adipokines. Catabolic factors produce synovitis, subchondral sclerosis, and joint injury (13).
Denoble suggests that uric acid is related to OA degradation through the inflammasome (NLRP3) (14). OA joints release nuclear agents that promote the crystallization of the urate. As OA progresses, cell death releases uric acid. In hyperuricemic patients, uric acid spreads from the blood to the synovial fluid in the joint. These diffusion mechanisms or apoptosis produce subacute inflammation and promote the progression of OA by activation of the inflammasome pathway (14). Similarly, Mc Alister states that NLRP3 (inflammasome) could be activated via the NF-?β pathway [nuclear factor-?β] or by caspases, releasing inflammatory cytokines (IL-1β and IL-18), leading to chronic inflammation, favoring OA progression (15). Wehmeyer states that OA and rheumatoid arthritis (RA) share the RankL [receptor activator of nuclear factor Kappa-β ligand] or NF-?β signaling pathway and that blocking this pathway would be a valid treatment option (16).
Pulsatelli states that OA is not only a disease of cartilage, but of subchondral bone and synovial tissue (17). Several signaling pathways act on chondrocytes, leading to cartilage alteration and bone formation in OA (16). Blocking these signaling pathways could prevent the progression of OA (17).
Kennedy states that inflammatory cytokines (IL-1β, IL-6, TNF-α) can act in several signaling pathways. They can release ROS and act on cartilage degradation. They may also act on MMPs in the matrix leading to the degradation of type X collagen. Inflammatory cytokines can also act on the RANKL membrane ligand, activating the NF-?β nuclear pathway, acting on the transcription factor SOX-9 (chondrogenic protein) and COX-2 (cyclooxygenase), degrading type II collagen (18).
Mobasheri states that not only the pathogenesis of OA is complex, but the phenotypes of OA are varied (19). There are several phenotypes in osteoarthritis (bone, metabolic, subchondral, aging-associated, cartilage-associated, trauma injury-associated, synovitis-associated, etc.); metabolism is involved in several of them, and they also share similar signaling pathways (19). In this regard, Berembaum suggests that the metabolic syndrome releases inflammatory mediators into the blood, which are harmful to the articular tissues and initiate or perpetuate this process. Once arthritic cells become active, they release inflammatory mediators in the joint and blood, amplifying low-grade inflammation and accelerating other low-grade chronic systemic diseases (20). Therefore, metabolic syndrome not only worsens OA, but also acute trauma, aging, and crystal diseases, releasing systemic mediators of inflammation that would exacerbate diseases such as Alzheimer’s disease, arteriosclerosis, or acute myocardial infarction (20). In summary, OA is affected by low-grade chronic inflammation as it occurs in metabolic syndrome (obesity, insulin resistance, lipid abnormalities, hypertension), and the development of OA contributes to low-grade chronic inflammation through the systemic effects of inflammatory mediators derived from OA, inducing and accelerating other chronic diseases (Alzheimer’s disease, stroke, myocardial infarction) (20).
Altered body composition, altered metabolites, and inflammation derived from fatty tissue and synovial tissue release inflammatory mediators that worsen OA. Abnormal dietary factors and dysfunctional fatty tissue produce and increase adipokines leading to an increased risk of OA development due to the release of inflammation mediators (complement, CRP, cytokines) (21).
Guisasola and Ortiz demonstrated an increase in inflammatory cytokines (IL-1β, IL-6, TNF-α and thermal shock proteins) in acute polytrauma (22). Perpetuation of an acute aggression leading to chronic inflammation may induce post-traumatic OA. Lieberthal states that resolving inflammation with specific cytokine blocking would solve the problem (23).
Single target treatment
Since there is no cure for OA, there are many therapeutic targets under study for the treatment of OA (24). The standard treatment in advanced OA is total replacement arthroplasty, with an efficiency of 95% at 10 years and 90% at 15 years (1). However, this approach involves risks and complications (1).
Many targets are proposed as therapeutic options for the management of early OA. Therapeutic targets include: A) mitochondrial function regulators, b) nutraceuticals, c) apoptosis regulators, d) iNOS [inducible nitric oxide synthase] inhibitors, e) analgesics and NSAIDs [non-steroidal anti-inflammatory drugs], f) MMP inhibitors, g) pro-inflammatory cytokine blockers, h) herbal medications, i) bone density conservation agents, j) bisphosphonates and k) strontium ranelate (24).
Nitric oxide inhibitors and antioxidants
Nitric oxide inhibitors and antioxidants are able to modulate inflammation, but are not useful in the management of OA (25,26).
Nutraceuticals
Curcumin could slow the progression of OA and decrease OA-related pain symptoms in the post-traumatic OA mouse model. Unfortunately, these findings have not yet been demonstrated in humans (27).
Uric acid reducing agents
OA, rheumatoid arthritis, and gout share similar signaling pathways. Unfortunately, medications lowering uric acid levels (such as allopurinol or uricosuric medications) do not lower the risk of total knee replacement (28).
Colchicine is an antimitotic and anti-inflammatory drug. In a recent study, colchicine showed a decrease in inflammation and biomarkers of bone turnover (both factors related to progression and severity in OA patients). However, in the long term (16-week follow-up), colchicine was not able to reduce the symptoms of OA of the knee (29).
Biological therapy
Biologic therapy targeting specific cytokines or interleukins (such as anakinra, infliximab, etanercept, adalimumab, tocilizumab, or denosumab) has shown to be effective in treating rheumatoid arthritis (RA). Unfortunately, biological drugs have not demonstrated their efficacy in the treatment of OA, there is no disease-modifying effect on the progression of OA (20). Biological drugs target inflammatory processes, but their excellent results observed in RA have not been successfully translated into OA (30).
Blocking IL-1
IL-1β is a cytokine related to inflammation and the catabolic process favoring the degradation and destruction of articular cartilage (30). Anakinra (an IL-1β antagonist) did not produce an improvement in OA symptoms compared to placebo (20,30).
Blocking TNF-α
TNF-α is a proinflammatory cytokine that targets chondrocytes causing cartilage loss (30). Infliximab showed initial tolerability in early exploratory trials, but was not effective in the treatment of knee osteoarthritis (20,30). Etanercept showed effectiveness in pain relief, but the effect lasted only 4 weeks (30). Adalimumab was safe and improved symptoms in OA, but has not progressed in the management of knee OA (30).
Multi-target treatment
Exercise and weight loss
Exercise increases the anti-inflammatory IL-10 at the perisynovial and intra-articular site of the knee (31). Weight loss decreases IL-6 and leptin and improves pain, function, quality of life, muscle strength, and endurance in patients with symptomatic knee osteoarthritis (32). Exercise and weight loss can act as a multimodal approach decreasing inflammatory cytokines and adipokines (IL-6, leptin) and increasing anti-inflammatory cytokines (IL-10) (31,32).
Hyaluronic acid
Hyaluronic acid (HA) is a component of synovial fluid and is responsible for its viscoelasticity. In OA, the concentration and molecular weight of HA decrease, reducing the mechanical protection of the joint. Intra-articular infiltration of HA may restore the elasticity of synovial fluid, adding shock absorption, lubrication, and joint protection. In addition, HA increased the proliferation of chondrocytes and decreased their apoptosis, slowing the destruction and progressive narrowing of the joint space, which is related to OA, acting as a chondroprotective agent. Anti-inflammatory and analgesic effects of HA have also been reported in the literature (33).
Nichols has stated that HA can act on ECM degradation and inflammation and pain (34). Depending on the molecular weight, HA may act on different cytokines of degradation and inflammation (34).
In the Nichols study, low molecular weight (LMW) HA inhibited MME (macrophage metalloelastase) and MMP-10 responsible for ECM degradation (34). Low molecular weight (LMW) HA also inhibited CCL-2 (chemokine ligand), CCL-3, CCL-4, CXCL-2 (chemokine receptor), CXCL-9, CXCL-10, and IL-12, responsible for the development of inflammation/pain (34). High molecular weight (HMW) HA inhibited ADAM-17 (disintegrin and metalloprotease), ADAMTS-4 (disintegrin and metalloprotease with thrombospondin motifs), ADAMTS-5 and MMP-1, MMP-2, MMP-3, related to ECM degradation. High molecular weight (HMW) HA also inhibited IL-6, NF-kβ (nuclear factor), phospho-Akt, phospho-JNK (c-Jun N-terminal kinase) and TLR-4 (toll-like receptor), cytokines related to the presence of pain and inflammation. Both LMW HA and HMW HA can act on MMP-9 and MMP-13, inhibiting ECM degradation. LMW HA and HMW HA inhibit IL-1β, IL-8, TNF-α, CCL-5, iNOS (inducible nitric oxide synthase) and COX-2 (cyclooxygenase), responsible for the presentation of pain and inflammation (34) (Table I).
Table I. Multitarget profile of hyaluronic acid, platelet-rich plasma and ozone with stimulation (↑) and inhibition (↓) effects.
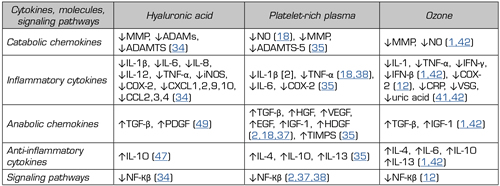
↓ = inhibition. ↑ = stimulation. MMMP: Matrix mineral metalloproteases. ADAM: disintegrin and metalloprotease. ADAMTS: Disintegrin and metalloprotease with thrombospondin motifs. NO: Nitric oxide. TNF-α: Tumor necrosis factor α. INOS: Nitric oxide synthase inducible. COX-2: Cyclooxygenase-2. CXCL: Chemokine receptor. CCL: Chemokine ligand. CRP: C-reactive protein. ESR: Erythrocyte sedimentation rate. TGF-β: Transforming growth factor β. HGF: Hepatocyte growth factor. VEGF: Vascular endothelial growth factor. EGF: Endothelial growth factor. IGF-1: Insulin growth factor 1. HDGF: Hepatocyte-derived growth factor. TIMPS: tissue inhibitor of metalloproteases. NF-kβ: Nuclear factor kβ.
HA acts on the symptomatic (pain and inflammation) and disease-modifying (ECM degradation) effects in OA, so HA may be a multi-target drug and a valid option for the treatment of knee OA (34).
Platelet-rich plasma (PRP)
Platelet-rich plasma is an autologous product that, by centrifugation, raises the platelet level severalfold compared to that observed in blood serum (30). PRP has several mediators communicating with the joint cells (30).
Some authors state that PRP contain 300 molecules or proteins identified by proteomics in the α-granules (35,36), while others argue that PRP contain nearly 800 proteins (37) and even 1200 proteins, including growth factors (GF) and anti-inflammatory cytokines (4). These GF and cytokines can inhibit inflammation and stimulate cartilage healing (4,30,35,36,37).
Kennedy states that PRP modulates inflammation by inhibiting IL-1β and TNF-α. PRP promotes cell proliferation and renewal by stimulating TGF-β (transforming growth factor β) and HGF (hepatocyte growth factor), VEGF (vascular endothelial growth factor), EGF (endothelial growth factor), IGF-1 and PDGF (platelet-derived growth factor) (18). In fact, HGF inhibits NF-kβ and decreases NO (nitric oxide) synthesis (18). Ornetti reported that PRP inhibits the NF-kβ pathway through HGF (37). PRP is also able of inhibiting the NF-kβ pathway via IGF-1 (37). HGF limits the inflammatory response within the synovial membrane (37). Demange stated that PRP can attenuate pro-inflammatory cytokines such as NF-kβ and IL-1 (38) (Table I).
PRP can act on different cytokines and signaling pathways, modulating inflammation and decreasing cartilage degradation and promoting cartilage healing (4,30,35,36,37,38). The multitarget profile of PRP makes this therapy a promising alternative for the treatment of knee osteoarthritis.
Ozone (O2-O3)
Ozone (O2-O3) modulates inflammation and pain in patients with knee OA (41). In addition, the anabolic effect of ozone (O2-O3) could play an important role in modifying the natural history of OA, as recently reported in a clinical case (39,40).
Recently, Fernandez-Cuadros et al. reported the immunomodulatory and anabolic properties of ozone (O2-O3) through the positive regulation of anti-inflammatory cytokines (IL-4, IL-10), growth factors (TGF-β, IGF-1), stem cells and inhibitory regulation of inflammatory and catabolic cytokines (MMPS, NO, PGE2] (1,40) (Table I).
Manoto et al. stated that ROS activate the NF-kβ pathway leading to cartilage apoptosis. The authors have also shown that ozone could block the NF-kβ pathway, decreasing inflammatory cytokines such as IL-1β, IL-6, TNF-α and COX-2, considering ozone (O2-O3) as a promising option for cartilage growth in patients with knee osteoarthritis (12). Fernandez-Cuadros has found that intra-articular ozone is capable of reducing CRP, ESR (erythrocyte sedimentation rate) and uric acid, recognized as biomarkers of inflammation in patients with OA of the knee, confirming that ozone modulates inflammation (41,42) (Table I).
From previous research, ozone (O2-O3) could be expected to play a role in the treatment of patients with knee OA (40,43).
RESULTS
This is the first article reviewing the role of inflammation as the leading cause in OA pathogenesis and suggests HA, PRP and ozone as potential treatment options due to their multitarget profile in inflammation modulation.
The pathophysiology of OA is very complex. Many signaling pathways are involved. Proteolytic molecules and inflammatory mediators are involved in the initial progression of the disease (2). OA was classically defined as a “wear and tear” disease, but now biochemical, biomechanical, metabolic, and genetic variables are considered key factors for disease progression (30), so the paradigm has shifted from biomechanical to inflammatory theory (3).
The therapeutic objectives in OA are: a) to slow the degradation-inflammation cycle, b) to inhibit inflammation mediators, c) to decrease catabolic chemokines, and d) to stimulate anabolic chemokines (35).
In this review, drugs that have a single-target treatment profile, including biological therapies (such as anakinra [anti IL-1β] or infliximab, etanercept, adalimumab [anti-TNF-α], tocilizumab [anti IL-6] and even denosumab [RANKL-A]), which have shown to be effective in treating RA by inhibiting specific inflammatory cytokines, have not shown to be equally effective in the treatment of knee OA (20).
For the treatment of knee osteoarthritis, recognized guidelines are established by the ACR (American College of Rheumatology), OARSI (Osteoarthritis Research Society International), EULAR and AAOS (American Academy of Orthopaedic Surgeons) (33). However, none of the clinical guidelines has yet included PRP or ozone as treatment options (30,33).
In the case of the HA, there is controversy over its use. The AAOS (2013 recommendation) and NICE (2014 recommendation of the National Institute for Health and Care Excellence) do not support its use; while OARSI (2014 recommendation) and ACR (2012 recommendation) are inconclusive regarding the use of HA (30). There is only one systematic review that has stated that intra-articular treatments showed the greatest effect on the management of knee OA, including PRP and HA with a molecular weight larger than 1500 kDa (44).
An updated meta-analysis and review published separately by Nori-Zadehh et al. and Vazquez et al. have defined ozone (O2-O3) as a valid option for the management of knee OA due to its effectiveness in pain management (45,46).
Three recent meta-analyzes (Chang 2013, Khoshbin 2013, Tietze 2014) have indicated that PRP offers better results compared with HA or corticosteroids, and the effect remains for at least 6 months (2). Ornetti and Andia, in two recent reviews, reported that PRP has a role in the treatment of knee OA because of its anti-inflammatory and regenerative properties and the clinical benefits have been confirmed in randomized and non-randomized controlled trials (36,37).
Zamboni has stated that AH and PRP can promote regeneration and reduce inflammation (47). HMW HA (50-120 kDa) added to PRP (combination infiltrate) reduces cytokines and chemokines responsible for OA progression (48). The combination of intra-articular injections of HA and PRP may decrease immune system-related cells and may recover cartilage degradation and may repair meniscus tears (49). In addition, HA has been shown to increase GF production if added simultaneously to PRP infiltration, reducing the time needed for healing. The combination of PRP plus HA enhances the release of TGF-1 and PDGF, favoring the healing effect (49).
In this review, HA, PRP and ozone have shown to have anti-inflammatory, immunomodulatory and regenerative properties due to their multitarget profile.
Three recent meta-analyzes confirm the usefulness of PRP and HA in the management of OA. Chevalier et al., in a meta-analysis reviewing 42 clinical trials, found that HA is more effective than corticosteroids in the management of OA (50). Tan et al, in a meta-analysis of 26 randomized and controlled studies, found that PRP is more effective than HA in the management of OA, although both treatments showed the same safety profile (51). Finally, Han et al., in a meta-analysis including 43 trials, found that HA demonstrated better outcomes than PRP in the treatment of knee OA (52).
For all of the above reasons, we pose HA, PRP and ozone as drugs with a multitarget profile for the management of knee OA, as has been described in this review.
CONCLUSIONS
OA is a complex pathophysiology disease involving cartilage, subchondral bone, and synovial tissue. New studies suggest that inflammatory mechanisms are involved in the pathogenesis of OA, even more than biomechanical factors, to the extent that this review has proposed a paradigm shift. The OA has no definitive cure today. The aim of treatment is to interrupt the vicious cycle of inflammation-degradation by blocking specific inflammatory and catabolic pathways. The option to treat a single target that blocks a single signaling pathway has not produced significant results in the treatment of knee OA, as seen in the treatment of rheumatoid arthritis. In addition to exercise and weight loss, the multi-target profile of ozone, PRP, and HA offer a promising alternative for the management of knee OA, as these alternatives may act on inflammatory cytokines and catabolic chemokines, and may stimulate anabolic chemokines and anti-inflammatory cytokines, as described above. Clinical guidelines may consider these medications for the treatment of knee osteoarthritis, given the biological properties described in this review.
CONFLICT OF INTEREST
The authors declare no commercial relationship that results in a conflict of interest in the conduct of this study.
FUNDING/SUPPORT
The authors note that no funding/support or fellowship was provided to perform this study.
REFERENCES