DOI: 10.20986/resed.2020.3775/2019
ORIGINAL
Intra-articular ozone modulates inflammation, ameliorates pain and rigidity, improves function and has anabolic effect on knee osteoarthritis: a prospective quasi-experimental before-and-after study, 115 patients
El ozono intrarticular modula la inflamación, mejora el dolor, la rigidez, la función y tiene un efecto anabólico sobre la artrosis de rodilla: estudio cuasiexperimental prospectivo tipo antes-después, 115 pacientes
M. E. Fernández-Cuadros1
O. Pérez-Moro1
M. J. Albaladejo-Florin1
S. Álava-Rabasa1
1Servicio de Rehabilitación y Medicina Física, Hospital Universitario Santa Cristina. Madrid, España
ABSTRACT
Purpose: The objective of the present study is to verify for the first time in the literature the symptomatic and modifying disease effect of ozone (O2-O3) through clinical (pain, function and stiffness), biochemical (C-reactive protein [CRP], erythrocyte sedimentation rate [ESR], uric acid) and radiological improvement (minimum medial and lateral joint space) in a series of patients with osteoarthritis of the knee.
Methods: A prospective quasi-experimental before-and-after study was performed in 115 patients with knee osteoarthritis Kellgren-Lawrence grade 2 or more. The ozone protocol consisted of 4 sessions (one session / week) of an intra-articular injection of 20 ml of a medical mixture of Oxygen-Ozone (95-5ºC) at a concentration of 20 µg / ml. Outcome variables included clinical (pain, stiffness, and function), biochemical (CRP, ESR, uric acid), and radiological variables (minimal femorotibial joint space).
Results: Mean age of the patients was 64.81 ± 11.22 years. Female patients accounted for 75.6 % (n = 87), with a female / male ratio of 3 : 1.
Biochemical-variables: CRP decreased from 0.42 ± 0.54 mg/dL to 0.31 ± 0.33 mg/dL (p = 0.0142). ESR decreased from 14.52 ± 10.14 mm/h to 13.08 ± 8.78 mm/h (p= 0.0014). Serum uric acid decreased from 5.12 ± 1.22 mg/dL to 5.05 ± 1.24 (p = 0.1307).
Clinical variables: Ozone (O2-O3) significantly improved pain, stiffness and function clinical variables (p = 0.0000). Pain measured by VAS was 7.11 ± 1.11 points and decreased significantly to 3.56 ± 1.56 points (p = 0.0000). Before the intervention, WOMAC-pain subscale was 14.3 ± 22.29 points and decreased to 7.13 ± 33.13 points (p = 0.0000), WOMAC-stiffness subscale was 2.73 ± 1.39 points and decreased to 1.16 ± 1.13 points (p = 0.0000), WOMAC-function subscale was 41.66 ± 8, 1 points and improved to 25.29 ± 9.72 points (p = 0.0000).
Radiological variables: In 53 patients analyzed radiologically (according to standardized protocol) at one year of follow-up after ozone treatment, the internal compartment increased significantly from 4.12 ± 1.41 mm to 4.4 ± 1.35 mm (p = 0.0008) and the external compartment increased from 6 ± 1.37 to 6.16 ± 1.4 mm (p = 0.0753).
Conclusions: Intra articular ozone has demonstrated a symptomatic and disease modifying effect in patients with osteoarthritis of the knee, improving pain, function and stiffness; decreasing markers of inflammation (CRP, ESR and uric acid), and increasing the minimal joint space of the medial and lateral component evidenced radiologically. In this study it has been shown that ozone modulates inflammation, decreases pain and stiffness, improves function and has an anabolic effect in patients with osteoarthritis of the knee. No adverse effect has been observed after intra articular infiltrations of ozone.
Key words: Ozone, biomarkers, pain, WOMAC, osteoarthritis, knee
RESUMEN
Objetivo: El objetivo del presente estudio es verificar por primera vez en la literatura el efecto sintomático y modificador de enfermedad del ozono (O2-O3) mediante la mejoría clínica (dolor, función y rigidez), bioquímica (proteína C-reactiva [PCR], velocidad de sedimentación globular [VSG], ácido úrico) y radiológica (mínimo espacio articular medial y lateral) en una serie de pacientes con artrosis de rodilla.
Material y métodos: Se realizó un estudio cuasiexperimental prospectivo tipo antes y después a 115 pacientes con artrosis de rodilla con Kellgren-Lawrence grado 2 o más. El protocolo de ozono consistió en 4 sesiones (una sesión/semana) de una infiltración intrarticular de 20 ml de una mezcla médica de oxígeno-ozono (95-5 %) a una concentración de 20 µg/ml. Las variables de resultado incluyeron variables clínicas (dolor, rigidez y función), bioquímicas (PCR, VSG, ácido úrico) y radiológicas (mínimo espacio articular femorotibial).
Resultados: La edad media de los pacientes fue de 64.81 ± 11.22 años. Los pacientes femeninos representaron el 75.6 % (n = 87), con una relación mujer/hombre de 3:1.
Variables bioquímicas: la PCR disminuyó de 0.42 ± 0.54 mg/dl a 0.31 ± 0.33 mg/dl (p = 0.0142). La VSG disminuyó sus valores desde 14.52 ± 10.14 mm/h hasta 13.08 ± 8.78 mm/h (p = 0,0014). El ácido úrico en suero disminuyó su valor de 5.12 ± 1.22 mg/dl a 5.05 ± 1.24 (p = 0.1307).
Variables clínicas: el ozono (O2-O3) mejoró significativamente las variables clínicas dolor, rigidez y función (p = 0.0000). El dolor medido por EVA fue de 7.11 ± 1.11 puntos y disminuyó significativamente a 3.56 ± 1.56 puntos (p = 0.0000). Antes de la intervención, la subescala WOMAC-dolor fue de 14.3 ± 2.29 puntos y disminuyó a 7.13 ± 3.13 puntos (p = 0.0000), la subescala WOMAC-rigidez fue de 2.73 ± 1.39 puntos y disminuyó a 1.16 ± 1.13 puntos (p = 0.0000), la subescala WOMAC-función fue de 41.66 ± 8.1 puntos y mejoró a 25.29 ± 9.72 puntos (p = 0.0000).
Variables radiológicas: en 53 pacientes analizados radiológicamente (según protocolo estandarizado) al año de seguimiento después del tratamiento con ozono, el compartimento interno aumento significativamente de 4.12 ± 1.41 mm a 4.4 ± 1.35 mm (p = 0.0008) y el compartimento externo aumentó de 6 ± 1.37 a 6.16 ± 1.4 mm (p = 0.0753).
Conclusiones: El ozono intrarticular ha demostrado efecto sintomático y modificador de la enfermedad en los pacientes con artrosis de rodilla, mejorando el dolor, la función y la rigidez; disminuyendo los marcadores de inflamación (PCR, VSG y ácido úrico), y aumentando el mínimo espacio articular del componente medial y lateral evidenciado radiológicamente. En este estudio se ha evidenciado que el ozono modula la inflamación, disminuye el dolor y la rigidez, mejora la función y tiene efecto anabólico en los pacientes con artrosis de rodilla. No se ha observado ningún efecto adverso tras las infiltraciones intrarticulares de ozono.
Palabras clave: Ozono, biomarcadores, dolor, WOMAC, artrosis, rodilla
Correspondence: Marcos Fernández-Cuadros
Correo electrónico: marcosefc@hotmail.com
Received: 28-10-2019
Accepted: 16-02-2020
INTRODUCTION
Osteoarthritis is the most prevalent joint disease. It affects nearly 4 million people and causes 50 % of total disability in Spain. Osteoarthritis affects the quality of life of people who suffer from it, physically, emotionally and socially. The economic impact is such that the direct cost of osteoarthritis in Spain is 4.738 million/year and represents 0.5 % of gross domestic product (1).
The healthy knee comprises articular cartilage, subchondral bone, synovial tissue, and articular capsule. In knee osteoarthritis there is destruction of the articular cartilage with narrowing of the articular space, sclerosis of the subchondral bone and formation of osteophytes and subchondral cysts; characteristics that are taken into account to classify knee osteoarthritis radiologically and its severity (1,2).
Osteoarthritis has no cure and its etiology is multifactorial. The short-term treatment objectives are to improve pain, function, and quality of life; and the long-term objective is to delay/reverse joint destruction. Conservative treatment includes hygiene and dietary measures, analgesics, NSAIDs, chondroitin sulfate/glucosamine, infiltrations (corticosteroids, hyaluronic acid, PRP). Definitive surgical treatment includes total replacement arthroplasty, which has a success rate of 95 % at 10 years, although this technique is not without risks and complications (2). Controversy exists regarding the symptomatic and disease modifying effect of conservative treatments, such as chondroitin sulfate/glucosamine (3,4).
Osteoarthritis has recently been linked to chronic low-grade inflammation, as chronic oxidative damage is believed to be involved in the changes and progression of knee osteoarthritis. According to authors such as Atías et al., Weinstein et al. and Borreli et al., chronic oxidative stress plays such an important role in knee osteoarthritis that the future of treatment will depend on the inhibition of oxidative damage without damaging the body’s antioxidant defense mechanisms (2). In this sense, it would be of great therapeutic value to act on the modulation and regulation of inflammation to decrease the progression of osteoarthritis. In addition, the future treatment of osteoarthritis should try to decrease cartilage destruction and promote joint repair. Therefore, the main objective in the management of osteoarthritis should be to act on a large number of biomarkers and/or proinflammatory cytokines produced in the affected joint (2).
Risk factors for osteoarthritis include obesity, trauma, biomechanical factors, and low-grade chronic inflammation (1,5). Several studies and years of experience have shown that ozone (O2-O3) is able to modulate inflammation and pain in patients with osteoarthritis of the knee (5). Furthermore, ozone (O2-O3) has anabolic effects that could modify the natural history of the disease in patients with osteoarthritis of the knee (5).
For the management of knee osteoarthritis, it is necessary to use biomarkers for the diagnosis, monitoring and progression of the disease. These biomarkers should assess clinical results (pain, stiffness, function), biochemical results (C-reactive protein [CRP], erythrocyte sedimentation rate [ESR], uric acid, interleukins) and radiological results (minimum femorotibial joint space) (6,7). However, as far as we know, there is no study that has evaluated the effectiveness of ozone in osteoarthritis of the knee taking into account analytical/biochemical or radiological biomarkers, but only clinical biomarkers.
The objective of this study is to verify for the first time in the literature the symptomatic and disease modifying effect of ozone (O2-O3) through clinical (pain, function and stiffness), biochemical (CRP,ESR) and radiological improvement (minimal medial and lateral joint space) in a series of patients with osteoarthritis of the knee.
MATERIAL AND METHODS
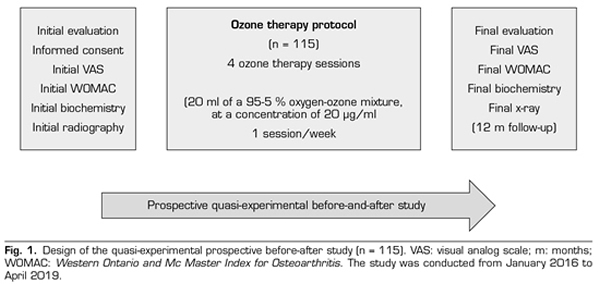
A prospective quasi-experimental before and after study was performed. A total of 115 patients with knee osteoarthritis with Kellgren-Lawrence (KL) grade 2 or more, who attended the Santa Cristina University Hospital, with clinical symptoms (pain, stiffness, loss of function), requiring conservative treatment, and in who previous symptomatic treatment failed were included in the study. The study was conducted from January 2016 to April 2019 and it was authorized by the Ethics Committee of the University Hospital of Santa Cristina, after signing an informed consent (Figure 1).

Inclusion criteria: 1) patients with osteoarthritis of the knee with a KL classification of grade 2 or more; 2) with pain larger than 3 on the visual analog scale (VAS) >3) in which any other conservative treatment has failed (NSAIDs, rehabilitation or physical therapy); 4) not willing or is not a candidate for replacement knee arthroplasty, 5) duly signed informed consent.
Exclusion criteria: 1) ozone allergy (O2-O3) (1); 2) incomplete biochemical analysis, either CRP, ESR, or uric acid; 3) incomplete treatment protocol; 4) absence of any of the applied questionnaires (VAS or Western Ontario and Mc Master Index for Osteoarthritis [WOMAC]); 5) absence of knee radiography before treatment and/or after one year of follow-up; 6) patients with favism (glucose 6-phosphate dehydrogenase enzyme deficiency) because it is an absolute contraindication for treatment with ozone.
Main objective: 1) assess the symptomatic and modifying effect of ozone disease using clinical, biochemical and radiological variables.
Secondary objectives: 1) assess the clinical variables pain, stiffness and function using the VAS and WOMAC scales; 2) assess the biochemical variables CRP, ESR and uric acid; 3) assess the radiological variables change of the tibiofemoral space of the medial and lateral compartments.
During the initial assessment, the treatment objectives, the procedure, the indications and contraindications were explained, the initial biochemical evaluation (analysis of CRP, ESR and uric acid) and the initial radiography of the knees were performed, the clinical scales (VAS and WOMAC) were provided and the informed consent was signed (Figure 1). We did not assess joint mobility in this study, because it was not considered as an outcome variable.
The ozone protocol consisted of 4 sessions (one session/week) of an intra-articular infiltration of 20 ml of a medical mixture of oxygen-ozone (95-5 %) at a concentration of 20 µg / ml. Skin was wiped with chlorhexidine 1 % and anesthetized with ethyl chloride before the infiltration. Ozonosan α-plus® was the medical generator used. This device provided a volume of 20 ml 95-5 % of oxygen and ozone mixture, that was introduced into a silicone-coated 20 ml syringe of 3 bodies. A 27G Quincke needle of 4 cm was used to deliver ozone (O2-O3) to the joint.
Once the patient lay down on the stretcher, the ozone medical mixture (O2-O3) was infiltrated in the knee, on the upper and lateral side of the patella. Immediately after the infiltration, knee flexion-extension movements were performed to favor the distribution of ozone in the joint, hearing the characteristic sign of intra-articular crepitus (Pérez-Moro maneuver) (1,6,7). In the event that the infiltration was not performed in the joint, crepitus was not heard in the joint, and redness and pain would appear after the infiltration; however, these complaints disappeared within a few minutes. The study authors performed the infiltrations of the treatment protocol.
After performing 4 sessions of the ozone protocol (O2-O3), the final evaluation was performed, biomarker analyzes, VAS and WOMAC scales were applied, and adverse effects (if any) were recorded. Serum levels of CRP, ESR and uric acid were measured at the beginning of the study and at the end of the protocol treatment.
Approximately 8 ml of venous blood was collected by venipuncture of the arm in a sterile vial in order to perform the analysis of the biochemical markers of inflammation (CRP, ESR and uric acid). A total of 5 ml of blood was collected in a tube without anticoagulant and serum was separated by centrifugation at 3500 rpm for 20 min. The serum CRP level was determined by the MULTIGENT CRP Vario assay (CRP VARIO, Italy). The minimum measurable concentration of CRP is approximately 0.2 mg/dl and its coefficient of variation is 5.8-6.3 % (6). Serum uric acid determination was performed using 3P39-41 Uric Acid Reagent Kit® (from Abbot, USA). The coefficient of variation of this determination is 3.6 % (7).
The rest of the blood sample (3 ml) was collected in another sterile tube containing potassium EDTA anticoagulant to measure ESR using the automated Ves-Matic Cube 30 system (Diese, Italy); although Westergren’s method is the reference method (6).
To classify knee osteoarthritis radiologically, the KL scale was used. KL grades are defined as follows: grade 0: without radiological changes; grade 1: doubtful osteophytes; grade 2: osteophytes are present; grade 3: a narrowing of the joint space is found; grade 4: presence of impingement, subchondral sclerosis, subchondral geodes and marginal osteophytes (8,9). The KL scale is the most used to classify knee osteoarthritis radiologically and constitutes a biomarker of its evolution (1,2).
Bilateral anteroposterior radiographs were performed, with both legs supported and fully extended, under load according to standardized protocol (9), for the radiographic evaluation of the medial and lateral tibiofemoral joint. All radiographic images were acquired digitally using a picture archiving and communication system (PACS). A total of 53 out of the 115 patients who completed one year of follow-up after the first infiltration were evaluated. Femorotibial distance was measured on radiograph in the medial and lateral compartments at the perceived distance as the narrowest of the joint space, and using the PACS measurement program. All evaluations were conducted by the same person, in order to reduce interobserver variation, whose coefficient of variation for repeated measures is 3-8 % (9,10).
Symptom severity was measured using the VAS and WOMAC scales. The VAS is a visual analogue scale for scoring pain from 0 to 10. A higher value is related to greater pain and vice versa (11). The WOMAC Index is a scale that assesses pain, stiffness, and function, all in 24 questions. Each answer has 5 possible options: none, mild, moderate, severe and extreme. Pain includes 5 items (rated from 0 to 20), stiffness includes 2 items (rated from 0 to 8) and function includes 17 items (rated from 9 to 68) (11). Any change in WOMAC index scores larger than 6 % is considered clinically important. These changes represent, for the WOMAC-pain subscale 1.2 points, for the WOMAC-stiffness subscale 0.5 points, and for the WOMAC-function subscale 4.1 points (12).
Statistical analysis was performed using SPSS® version 20.0. Frequencies and percentages were used to evaluate qualitative variables; while means and standard deviation were used for the evaluation of quantitative variables. The T-Student test was the tool used to evaluate any change before and after treatment in the quantitative variables. The significance level was 95 % (p < 0.05). Although it was not the aim of the study, the patients were systematically evaluated every six months, because we know that the effect of the treatment diminishes after 6 months, according to our experience and studies previously published by our study group(1,5,6,7,8,9) in order to restart treatment in the cases that are necessary.
RESULTS
115 patients were evaluated in this study. The mean age of the patients was 64.81 ± 11.22 years. Female patients accounted for 75.6 % (n = 87), while male patients accounted for 24.4 % (n = 28), with a female: male ratio of 3:1 (Table I).
The most frequent radiological grade was KL grade 2 (n = 71; 61.7 %), followed by KL grade 3 (n = 34; 29.5 %) and KL grade 4 (n = 10; 8.6 %) (Table I).
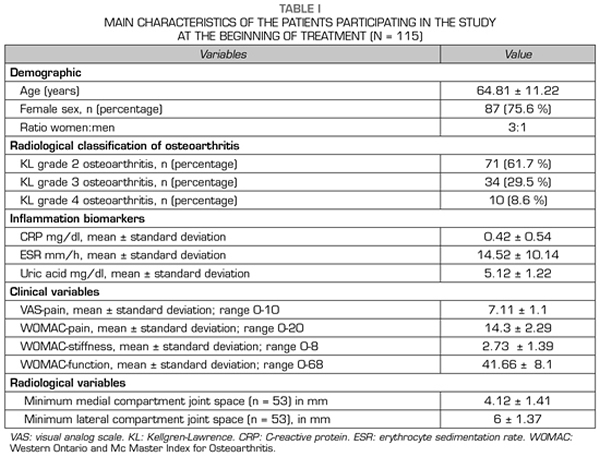
With regard to the outcome variables, the CRP and ESR inflammation biomarkers decreased significantly (p < 0.05) after ozone therapy (O2-O3). The PCR decreased from 0.42 ± 0.54 mg/dl to 0.31 ± 0.33 mg/dl (p = 0.0142) (Table II). The ESR decreased its values from 14.52 ± 10.14 mm/h to 13.08 ± 8.78 mm/h (p = 0.0014) (Table II). Serum uric acid decreased its value from 5.12 ± 1.22 mg/dl to 5.05 ± 1.24 (p = 0.1307) although this decrease was not statistically significant (Table II).
With regard to the severity of symptoms (pain, stiffness and function) in knee osteoarthritis, measured by using VAS and WOMAC scales, ozone therapy (O2-O3) significantly improved every one of the variables (p = 0.0000). Before treatment, the pain measured by VAS was 7.11 ± 1.11 points and decreased significantly to 3.56 ± 1.56 points (p = 0.0000) (Table II). Before the intervention, the WOMAC-pain subscale was 14.3 ± 2.29 points and decreased to 7.13 ± 3.13 points (p = 0.0000), the WOMAC-stiffness subscale was 2.73 ± 1.39 points and decreased to 1.16 ± 1.13 points (p = 0.0000), the WOMAC-function subscale was 41.66 ± 8.1 points and improved to 25.29 ± 9.72 points (p = 0.0000) (Table II).
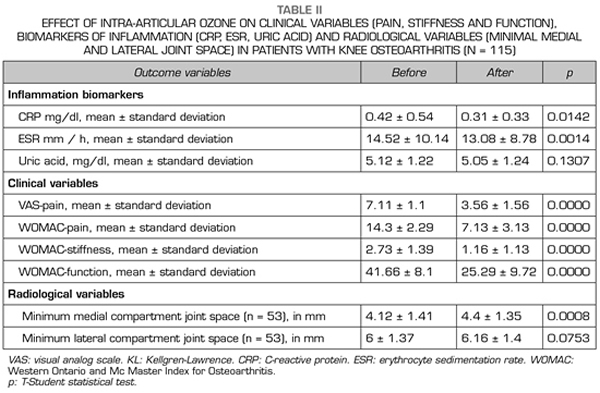
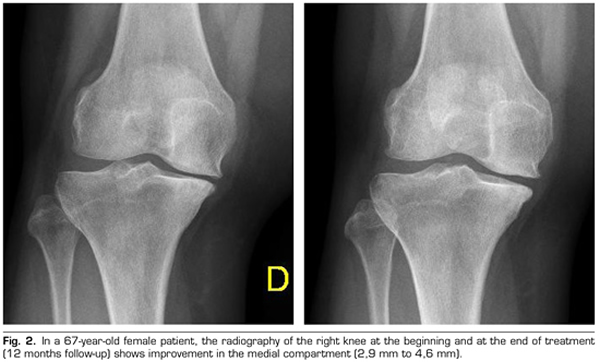
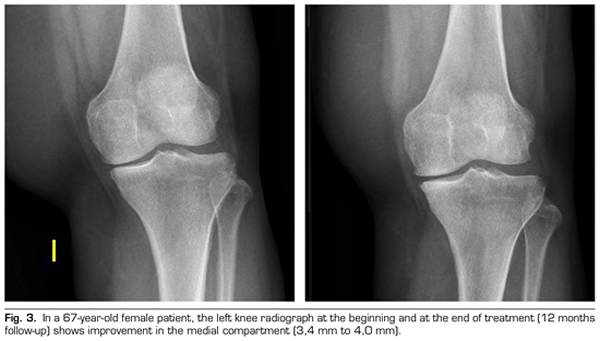
Regarding the radiological variables, when evaluating 53 out of the 115 patients who completed one year of follow-up after ozone treatment, it was observed that the internal compartment increased significantly by 4.12 ± 1.41 mm to 4.4 ± 1.35 mm (p = 0.0008) and the external compartment increased from 6 ± 1.37 to 6.16 ± 1.4 mm (p = 0.0753) (Table II). Two clinical cases are presented as a sample of the radiological change in the internal and external compartments (Figures 2 and 3).
No adverse event has been reported after treatment, except pain after infiltration, which subsided within a few minutes.
DISCUSSION
As far as we know, this is the first article that has demonstrated the effectiveness of ozone (O2-O3) in osteoarthritis of the knee by modifying clinical, biochemical and radiological biomarkers. This article confirms the symptomatic and disease modifying effect of intra-articular ozone therapy in a series of patients with osteoarthritis of the knee.
Knee osteoarthritis is the most common cause of pain and disability in western societies, such as in Spain (2). It is so prevalent that 50 % of people with ages between 60-75 years have some radiological sign, and 80 % of people over 80 show clinical and radiological signs (1,2).
Osteoarthritis commonly affects middle-aged people. In younger groups, osteoarthritis affects both sexes equally, but in people over 50 years old women are more affected, as in our study (female/male ratio was 3/1), and with a mean age of 64.81 (1,2,5) years.
At the moment, no treatment for osteoarthritis is available. Therefore, the management objectives are to decrease symptoms (pain, stiffness, dysfunction) and decrease/slow down joint wear and tear and destruction (1,2), objectives that have been achieved in the patients in this study treated with ozone (O2-O3).
Malathi et al. suggest that the development of osteoarthritis is often accompanied by inflammation (13). Various epidemiological studies suggest that the severity and progression of tibiofemoral joint cartilage loss is more frequent and severe in patients with inflamed synovial fluid (14). Higher levels of IL-1β and TNF-α, which are mediators of inflammation, are found in the progression of osteoarthritis (13). Other studies have reported that high levels of CRP are related to the prevalence and progression of knee or hip osteoarthritis. (13) Other authors report that elevated levels of CRP are related to IL-6 levels in synovial fluid and synovial infiltration, as well as symptoms of pain and stiffness, radiological classification, and progression of osteoarthritis (15). Many other researchers in the world have suggested that not only IL-6, but also CRP and ESR are closely related to radiological osteoarthritis of the knee, the severity of symptoms and the progression of the disease (16,17,18,19). These biomarkers indicate that low-grade inflammation could be a factor involved in the structural and symptomatic changes in knee osteoarthritis (20). Furthermore, the data presented shows that inflammatory biomarkers, and in the particular case of this study, CRP and ESR, could be a key factor in the pathogenesis of osteoarthritis of the knee and could be used as predictors and outcome variables (6).
Regarding uric acid, several epidemiological studies have reported an association of uric acid with systemic inflammation and with osteoarthritis. Roddy and Doherty suggest that gout and osteoarthritis share a common pathogenic link (21). Ma and Leung state that the deposition of monosodium uric acid crystals secondary to hyperuricemia promotes the direct degradation of cartilage (22). Martinon et al. and Denoble et al., in different studies, reported that monosodium uric acid crystals activate macrophage innate immune responses through Natch Domain, Leucin-rich repeat, and pyrin domain containing protein 3 (NALP3) activating caspase-1 and releasing IL-1β and IL-18, cytokines related to cartilage degradation (23,24). According to these authors, gout and osteoarthritis would share the same inflammatory pathway (22,23,24). Several demographic studies in healthy men and women demonstrated that serum uric acid is positively associated with CRP (25). A study analyzing 957 elderly Italian people showed that serum uric acid is positively associated not only with CRP, but also with TNF-α and IL-6 (26). In another study including 608 Swiss Caucasian individuals, serum uric acid was found to be positively associated with CRP, TNF-α, and IL-6 (in both men and women) (27). Billiet et al. mentioned that uric acid can stimulate the production of TNF-α in synovial cells (28).
Due to all these considerations, CRP, ESR and uric acid are considered inflammatory biomarkers involved in the pathogenesis of osteoarthritis, and have been evaluated in this study.
In a recent review of the literature, Fernández-Cuadros et al. stated that ozone (O2-O3) is able to modulate inflammation, acting on various inflammation markers, inhibiting proinflammatory cytokines, minerometalloproteases, nitric oxide, prostaglandin E2 and stimulating anti-inflammatory cytokines (1,2). Furthermore, Fernández-Cuadros et al. have also reported that ozone (O2-O3) is able to stimulate growth factors (TGF-1, IGF-1), chondrocytes and stem cells (1,2). For these reasons, we believe that ozone (O2-O3) could modulate inflammation and have an anabolic effect on knee osteoarthritis, this hypothesis has been demonstrated in this study.
In our study, ozone (O2-O3) has been able to decrease inflammation markers such as CRP (from 0.41 to 0.31mg %), ESR (from 14.52 to 3.08 mm/h) and uric acid (from 5.12 mg % to 5.05 mg %), confirming that ozone (O2-O3) is able to modulate inflammation in patients with knee osteoarthritis, decreasing these inflammation markers. This finding is related to what was recently published by our study group (6,7).
In this study, ozone (O2-O3) has been able to decrease pain and stiffness, in addition to improving function and quality of life, evidenced by an improvement in the VAS and WOMAC clinical scales, with a duration of effect 6 months. These results are consistent with what was previously reported by our study group (1,6,7,8). The symptomatic effect of ozone (O2-O3) on knee osteoarthritis is demonstrated in this study.
Radiography is usually the initial imaging examination performed in patients with osteoarthritis of the tibiofemoral joint. Radiography is also commonly used in demographic studies to define the presence of osteoarthritis of the tibiofemoral joint and to document changes in the severity of the history of the disease over time (1). The articular cartilage becomes thinner and inflamed as osteoarthritis progresses, but the thickness of the cartilage normally decreases over time in knee osteoarthritis (2). Progression is greater in the medial compartment compared to the lateral compartment of the tibiofemoral joint (2). Based on this assumption, there is a moderate correlation between the narrowing of the joint space and the loss of articular cartilage (2,3,4,14). Joint space narrowing is defined when the minimum width of the joint space is less than 3 mm for the tibiofemoral joint (29). Since cartilage is not observed on radiography, its loss is indirectly measured by the narrowing of the joint space (9). Therefore, radiography is an inexpensive method to monitor the progression of osteoarthritis and, currently, it is the “gold standard”, that is, the simplest and most accepted method of evaluating the progression of osteoarthritis and cartilage destruction (30,31,32,33,34,35). A change in the minimum tibiofemoral space is considered as a primary measure of biological change in osteoarthritis, and indirectly, a biomarker to evaluate biological treatments in osteoarthritis (36).
After a year of follow-up with radiological controls on 53 out of the 115 patients in the study, ozone (O2-O3) has been able to increase the minimum joint space of the medial compartment (from 4.12 to 4.44 mm) and the lateral compartment (from 6.0 to 6.16 mm), reversing knee osteoarthritis in our case series. This finding suggests the disease modifying effect of ozone (O2-O3) on knee osteoarthritis, findings that is consistent with the recently reported by Fernández-Cuadros et al. (9).
As far as we know, this is the first article that radiologically suggests the anabolic or structural effect of ozone on knee osteoarthritis, and therefore, modifier of the disease. There is only one study that has reported a disease modifying effect on osteoarthritis of the knee, through the oral use of glucosamine/chondroitin sulfate (3), although despite that study reported that such treatment only achieved anabolic effect on the lateral compartment and no anabolic effect was found in the medial compartment, it mainly refers to the natural evolution of the disease (osteoarthritis) than to a real modification produced by such drugs on knee osteoarthritis (4).
In our study, and based on our experience, we have applied 20 ml of ozone at a concentration of 20 m g/ml in all patients (1,5,6,7,8,9). It is likely that the greater amount of ozone (400 m g total dose) was responsible for the prompt effectiveness observed at the end of treatment (at 4 weeks), although we have not compared it with another group that received less volume or lower dose/concentration. It is important to note that we have applied only 4 sessions in our study, while other studies apply up to 8-12 sessions (37,38,39,). This has an economic and logistical impact, since health resources are limited. In our study, we assessed pain, stiffness, and function using the VAS and WOMAC scales, as do most of the studies known to date (37,38,39,40). In addition, we have treated patients with knee osteoarthritis KL grade 2 to 4, while most studies treat patients with KL grade 1-2 and KL grade 2-3 (37,38,39,40).
Thus, in comparison with our study, Lopes de Jesús (2017) treated patients with KL grade 2-3, evaluated them with the VAS and WOMAC scales and applied intra-articular ozone, 10 ml × 20 m g/ml, 8 sessions (once per week) (37,38,39,40). Reissadat (2018) treated patients with KL grade 2-3, evaluated them with the VAS and WOMAC scales and applied intra-articular ozone, 10 ml × 30 m g/ml, 3 sessions (once per week) (37,38,39,40). Hashemi (2015) treated patients with KL grade 2-3, evaluated them with the VAS and WOMAC scales and applied intra-articular ozone, 10 ml × 40 m g/ml, 8 sessions (3 times the first week, 2 times the second week and once per week for 3 weeks) (37,38,39,40). Babaei-Ghazani (2018) treated patients with KL grade 2-3, evaluated them with VAS and WOMAC scales and applied intra-articular ozone 10 ml × 15 m g/ml, 1 single dose (37,39,40). Feng (2017) treated patients with KL grade 3-4, evaluated them with the VAS and Lysholm scales and applied intra-articular ozone, 20 ml × 20 m g/ml, 12 sessions ( twice per week for 6 weeks) (37,39). Calunga (2012) treated patients with knee osteoarthritis without specifying KL grade, evaluated them with the VAS and applied rectal ozone for 20 sessions, 100-200 ml × 25-40 m g/ml and -intra-articular ozone, 5-10 ml × 30 m g/ml, 4 sessions (twice per week) (37). Duymus (2017) treated patients with KL grade 2-3, evaluated them with the VAS and WOMAC scales and applied intra-articular ozone, 15 ml × 30 m g/ml, 4 sessions (once per week) (37,38,39,40). Hashemi (2017) treated patients with knee osteoarthritis without specifying a KL grade, evaluated them with the VAS scales and assessed the IL-1β and TNF-α biomarkers, and applied intra-articular ozone, × 35 m g/ml, 1 single dose ) (37,38,39,). Hashemi (2016) treated patients with KL grade 2-3, assessed them with the VAS and Oxford Knee Scales and applied intra-articular ozone 10 ml × 40 m g/ml and periarticular ozone 5 ml × 10 m g/ml per point, 8 sessions (3 times the first week; 2 times the second week and once per week for 3 weeks) (37,38,39). Fernández-Cuadros (2018) treated patients with KL grade 2-4, evaluated them with the VAS and WOMAC scales and applied intra-articular ozone, 20 ml × 20 m g/ml, 4 sessions (once per week) (7,37). Mishra (2011) treated patients with KL grade 2, evaluated them with the WOMAC and modified MacNab scales and applied intra-articular ozone, 10 ml × 30 m g/ml, 3 sessions (once per month) (38,39). Gombini (2016) treated patients with KL grade 2-3, evaluated them with the VAS and Oxford Knee Scale and applied intra-articular ozone, 15 ml × 15 m g/ml, 5 sessions (once per week) (38). Chansoria (2016) treated patients with KL grade 1-2, evaluated them with the VAS and WOMAC scales and applied intra-articular ozone, 5 ml × 25 m g/ml, a single dose (38,39). Finally, Invernizzi (2017) treated patients with KL grade 2-3, evaluated them with the VAS and Oxford Knee Scale, SF-12 and EUROQoL scales and applied intra-articular ozone, unspecified volume at a concentration of 20 m g/ml, 4 sessions (once per week) (38,39). All these studies have shown clinical improvement with ozone therapy in osteoarthritis of the knee, measured by the scales used, which is consistent with what was observed in our study. Only one study (Hashemi 2017) has evaluated proinflammatory cytokines IL-1β and TNF-α (37,38,39). No study has evaluated the biomarkers CRP, ESR, uric acid, or has radiologically evaluated the evolution after intra-articular ozone therapy (37-40).
In our study, the use of intra-articular ozone (O2-O3) has been shown to be a safe therapy, without adverse effects and capable of objectively improving clinical, analytical and radiological biomarkers. Therefore, our results are consistent with those described by Arias-Vazquez et al., Noori-Zadeh et al., Sconza et al. and Oliviero et al. who in recent systematic reviews and meta-analyzes, in addition to mentioning our previous publications (5,7,8) highlighting the importance, relevance and timeliness of our findings, they believe that ozone (O2-O3) should be considered as a therapeutic alternative for the management of osteoarthritis of the knee, due to its proven effectiveness (37,38,39,40).
CONCLUSION
Intra-articular ozone has shown a symptomatic and disease modifying effect in patients with osteoarthritis of the knee, improving pain, function and stiffness; decreasing the markers of inflammation (CRP, ESR and uric acid), and increasing the minimal joint space of the medial and lateral component evidenced radiologically. In this study, it has been shown that ozone modulates inflammation, decreases pain and stiffness, improves function and has an anabolic effect in patients with osteoarthritis of the knee. No adverse effect has been found after intra-articular infiltrations of ozone.
CONFLICTS OF INTEREST
We declare that authorization is provided for the transfer of all copyrights on the publication to RESED, as well as the non-existence of a conflict of interest of any of the authors.
SOURCES OF FUNDING
There is no funding for the conduct of this study.
ACKNOWLEDGMENTS
To Saturnino Díaz Trujillo, librarian of the Santa Cristina University Hospital, Madrid, for the literature search to conduct this study. To the assistants Aurelia Gómez Sierra and María del Carmen de la Riva Molina, for the logistical support in conducting this study.
BIBLIOGRAFÍA