
DOI: 10.20986/resed.2020.3801/2020
REVISION
Opioid misuse in patients with cancer pain: an integrative systematic review of the literature
Uso inadecuado de opioides en pacientes con dolor oncológico: revisión sistemática integradora de la literatura
M. Labori Trias1, R. Hernández-Ribas2 and J. Porta-Sales1,3
1Servicio de Cuidados Paliativos, Institut Català d’Oncologia. Hospitalet de Llobregat, Barcelona, España
2Servicio de Psiquiatría, Hospital Universitari Bellvitge. Institut Català d’Oncologia. CIBERSAM. Universitat de Barcelona, España
3Universitat Internacional de Catalunya. Barcelona, España
Labori Trias M, Hernández-Ribas R, Porta-Sales J. Opioid misuse in patients with cancer pain: an integrative systematic review of the literature. Rev Soc Esp Dolor. 2020;27(5):1-0
Received: 12-03-2020
Accepted: 06-06-2020
Correspondence: Maria Labori Trias
mlabori@iconcologia.net
ABSTRACT
Background: Opioids are widely used for the treatment of cancer pain and non-malignant pain. There is a lot of information about opioid misuse (OM) in patients with non-malignant pain, however in cancer patients there is less evidence.
Objectives: To identify, appraise and synthesize existing evidence about epidemiology, risk factors, clinical tools and evolution of OM in patients with cancer pain.
Methods: Integrative systematic review with data extraction and narrative synthesis. PubMed, Web of Science y PsychINFO databases were searched for articles published through 31 December 2017. Study inclusion criteria were as follows: 1) published in English, Spanish or French language; and 2) containing data on the prevalence or incidence of OM in patients with cancer pain; or/and 3) providing information about OM risk factors, mortality, duration and remission.
Results: The search yielded 3520 articles, of which 40 met the inclusion criteria. Four themes were identified: 1) epidemiology, 2) risk factors, 3) patient’s and professional’s opinion, and 4) specific policies. The obtained results were very heterogeneous; the prevalence of OM varied from 0 up to 26 % and the prevalence of opioid-associated aberrant behaviours from 12 to 85 %. Different risk factors for OM were identified, highlighting young age and history of psychiatric disorders or substance abuse, and different tools for risk assessment or diagnosis of OM were described. Regarding professional’s opinion, it seems to be more awareness about OM but nevertheless policies regarding this problem are scarce.
Conclusions: Even though there is a greater awareness among professionals about OM in cancer patients, the current information is very heterogeneous and does not allow clear conclusions. For this reason, it will be necessary to carry out new studies trying to standardize criteria and establish better protocols and policies for detection and management of OM.
Keywords: Cancer pain, analgesics opioids, opioid-related disorders, epidemiology, risk factors.
RESUMEN
Introducción: Los opioides son ampliamente utilizados para el control del dolor oncológico y no oncológico. Existe mucha información sobre el uso inadecuado de opioides (UIO) en pacientes no oncológicos, sin embargo en pacientes oncológicos existe menos evidencia.
Objetivos: Identificar, analizar y sintetizar la evidencia disponible sobre la epidemiología, los factores de riesgo, instrumentos clínicos y evolución del UIO en pacientes con dolor oncológico.
Material y métodos: Revisión sistemática integradora de la literatura con extracción de datos y síntesis narrativa. Las fuentes utilizadas para buscar artículos publicados hasta el 31 de diciembre de 2017 fueron las bases de datos PubMed, Web of Science y PsychINFO. Se eligieron aquellos artículos que siguieran los siguientes criterios de inclusión: 1) publicados en lengua inglesa, española o francesa, y 2) que incluyesen información sobre la prevalencia o incidencia del UIO en pacientes con dolor oncológico, o/y 3) que aportasen información sobre factores de riesgo, mortalidad, duración del UIO y su remisión.
Resultados: De la búsqueda surgieron 3520 artículos, de los cuales 40 cumplieron los criterios de inclusión. Se identificaron cuatro áreas temáticas: 1) epidemiología, 2) factores de riesgo, 3) opinión de pacientes y profesionales, y 4) políticas específicas. Los resultados obtenidos fueron muy heterogéneos, identificándose una prevalencia que oscila entre el 0 y el 26 % en el UIO y entre el 12 y el 85 % en las conductas aberrantes relacionadas con opioides. Se identificaron diferentes factores de riesgo de UIO, destacando la edad joven y la presencia de antecedentes psiquiátricos o de abuso de sustancias, y se describieron diferentes instrumentos dirigidos a la evaluación del riesgo o bien al diagnóstico del UIO. En cuanto a la opinión de los profesionales, parece haber un incremento de la sensibilización al respecto, pero las políticas de los centros en relación con esta problemática suelen ser casi inexistentes.
Conclusiones: A pesar de que existe una mayor sensibilización de los profesionales acerca del UIO en pacientes oncológicos, la información de que disponemos es muy heterogénea y no nos permite extraer conclusiones claras. Por esta razón será necesaria la realización de nuevos estudios intentando homogeneizar criterios y establecer mejores protocolos y políticas de detección e intervención frente al UIO.
Palabras clave: Dolor oncológico, opioides, uso inadecuado, epidemiología, factores de riesgo.
INTRODUCTION
It is widely known that opioids are substances capable of producing addictive disorders, as is the case with other drugs such as alcohol, benzodiazepines, or cocaine. The latest edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) describes “opioid use disorder” as the set of signs and symptoms reflecting prolonged and compulsive self-administration of opioids that are not used for legitimate medical purposes, or if there is another medical condition requiring treatment with opioids, by using it in doses far greater than the amount needed for that medical condition (1). If we focus only on medically prescribed opioid-related disorders, and independently of DSM-5, the concepts of “opioid misuse” have been well accepted in the literature that it could be defined as the use of such medication in a manner other than that prescribed in the therapeutic plan, and “aberrant opioid-related behavior”, which would include any behavior that reflected misuse (2,3). The presence or absence of aberrant behavior in the consumption of prescribed opioids is a key point in assessing the clinical response to such treatment and is, in fact, part of the calls 4A’s that define the success of opioid treatment: 1) the analgesia obtained; 2) the possibility of performing the activities of daily life; 3) the absence of adverse effects; and 4) the absence of aberrant opioid-taking behaviors (4). “aberrant” behaviors would in themselves include different situations, defined in English as: “misuse,” “abuse,” “addiction,” “diversion,” and “chemical coping” (2,3,5) (see definitions in Table I). Chemical-coping behavior, which may or may not be part of an addiction, and which is defined as consumption above the recommended limits for anxiolytic purposes and accompanied by compulsive and destructive behaviors, deserves special attention. In the case of cancer patients, such chemical-coping behavior may aim to manage the anxiety caused by the disease and the pain (5).
TABLE I. DIFFERENT SITUATIONS OF OPIOD USE INCLUDED INT HE CONCEPT OF "ABERRANT" BEHAVIOR (3)

One of the central aspects of opioid misuse is its clinical assessment and, in this regard, several evaluation tools have been developed. Existing instruments to date could be divided into two large groups: Those aimed at estimating the risk of non-compliance with opioid prescription, the most widely used are the “CAGE-AID”, modification of the CAGE questionnaire aimed at the detection of problematic use of drugs such as opioids (6), the “Opioid Risk Tool” (ORT) (7), and the “Screener and Opioid Assessment for Patients with Pain” (SOAPP) (8). And those focused on the detection and monitoring of the already established misuse, where the “Current Opioid Misuse Measure”(9) would be highlighted. It is important to point out that, as of today, none of these tools are validated in Spanish.
As regards the prevalence of opioid misuse, it should be noted that in the last decade several authors (10-12) and entities, such as the U.S. Department of Justice (13), have drawn attention to the increase, close to 150%, in opioid use and misuse, especially in patients with chronic noncancer pain in the United States (USA). This fact also conditions an overall increase in associated health costs without evidence of clinical benefit, and an increase in opioid-related comorbidities. In the U.S. an increase of up to 43 % in opioid misuse has been estimated in the first decade of this century, with a parallel increase in unintentional opioid deaths (14). In Canada, situations similar to those in the US has been described, among all drug-related deaths in Ontario between 2006 and 2008, 58 % were reported to be associated with opioid use, mostly (93 %) in non-cancer patients (15). Progressively, this alarm has spread to Europe (16), and in recent years studies have begun to address patients with cancer pain (17).
In Spain, in response to this general concern, the “Consensus Guide for the Good Use of Opioid analgesics” was published in 2017, as the result of the work of four scientific societies: Socidrogalcohol, the Spanish Society of Family and Community Medicine (SEMFyC), the Federation of Community Nursing and Primary Care Associations (FAECAP) and the Spanish Society of Palliative Care (SECPAL)(18). This guide attempts to provide standard guidelines for action, both at the prevention level and at the global level, for the misuse of medically prescribed opioids.
In any case, and despite the reasonable alarm, it is important to point out that the vast majority of available information refers to the use of opioids in patients with chronic pain of non-cancer origin. In fact, studies analyzing the misuse of prescribed opioids in patients with cancer describe it as very rare, whether in the management of baseline and breakthrough pain (19-21) The first two reviews regarding cancer patients found that the information available was inconclusive, with the prevalence of opioid addiction ranging from 0% to 7.7 %, based on the studies analyzed (22,23). However, a more recent review concludes that one in five cancer patients would be at risk of misuse of prescribed opioids, a number of large clinical relevance that advises to deepen the study of this process (17).
For all of the above, we believe that further evidence is needed on the potential opioid misuse in cancer patients. Thus, the objective of the present review is to systematically review and synthesize available evidence on the frequency, type, and risk factors associated with the opioid misuse in patients with cancer pain.
MATERIAL AND METHODS
Design and source of data
A systematic review has been performed following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guide (24). The literature was searched in the following databases: PubMed, Web of Science and PsychINFO, evaluating all publications from its set up until December 31, 2017.
Eligibility criteria
Articles in English, Spanish, or French including information on the prevalence or incidence of opioid misuse in patients with cancer pain and/or providing information on risk factors, mortality, duration of misuse, and remission were considered eligible. No editorial or letters to the director, case series, or non-systematic reviews were included.
Search strategy and study selection
The search strategy for the PubMed database, which uses both MeSH terms and non-MeSH keywords, is shown in Annex 1 (https://www.resed.es/Documentos/ArticulosImg/1022/07_rev_labori_usos_ing_anexo1.jpg). Following the same model, the search strategy was adapted for each database. The articles identified followed a selection process conducted by three reviewers (M. L., R. H-R. and J. P-S.) in three phases: Initially by title, then by abstract and finally by full text. In addition, a manual search of the references of the articles identified was conducted. Articles that did not meet the above inclusion criteria were excluded and, in case of doubt, an agreement between the three reviewers was reached. Figure 1 shows the process of searching and selecting the articles.
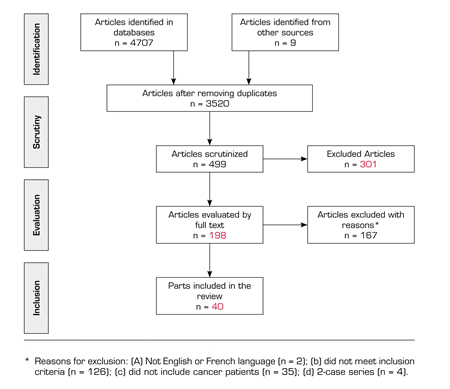
FIGURE 1. PRISMA FLOW CHART OF STUDY SELECTION
Data extraction and analysis and synthesis of the results
The data were extracted and synthesized using the integrative method described by Whittemore and Knafl (25), which allows the synthesis of various sources including both empirical studies (qualitative, quantitative and mixed methods) and theoretical studies (cases, theoretical models, comments and reflections). The process of analyzing the data using this methodology allows the integration of the findings of all the included studies. All studies that met the inclusion criteria were treated in the same way in the present review. The data analysis process proposed by Whittemore and Knafl (25) was also used, which includes the synthesis, presentation and comparison of the data, as well as drawing conclusions. The first step is to develop a matrix to extract the main characteristics of each of the articles included: Author/s, year of publication, country where the study was performed, type of study, number of patients included, main objective, opioid/s evaluated, and main results. Following the analysis of the matrix, four themes related to the objectives of the present review emerged from data: 1) prevalence/incidence, 2) risk factors and assessment tools, 3) opinion of professionals and patients, and 4) specific policies on the opioid misuse that were implemented. All articles that met the inclusion criteria were then classified into one of the above-mentioned four categories and they were analyzed. The data for each article were extracted and compiled in the data matrix, regardless of the level of evidence they provided. The third step was point-by-point comparison of the extracted data to ensure that similar data were compared, categorized, and synthesized. Data was manually managed to organize and facilitate analysis.
Quality analysis
The PRISMA guideline was followed to communicate the characteristics of the studies and their methodological quality (24). Due to the lack of consensus in the literature regarding the exclusion of studies for methodological reasons (26), no study was excluded due to quality issues in the present review.
RESULTS
A total of 198 out of the 3520 articles initially identified were reviewed in full text, 167 of them were excluded (see reasons for exclusion in Figure 1). Forty publications met the inclusion criteria and were included in the review. Annex 2 (https://www.resed.es/Documentos/ArticulosImg/1022/07_rev_labori_usos_eng_anexo2.jpg) shows the main characteristics of the 40 selected articles with a summary of the main findings. The methodologies of the articles included in the review are diverse: Systematic reviews (n = 2; 5 %), retrospective studies (n = 12; 30 %), prospective studies (n = 8; 20 %), cross-sectional studies (n = 15, 37,5 %), qualitative studies (n = 2; 5 %) and Delphi studies (n = 1; 2.5 %). Twenty-two (55 %) articles have been published in the USA, 9 (22.5 %) in European countries, 3 (7.5 %) in Asian countries, 1 (2.5 %) in Egypt, 1 (2.5 %) in Australia, 1 (2.5 %) in Brazil and 3 (7.5 %) are multi-national studies. Regarding the type of opioid analyzed in the different studies, it should be noted that all opioids are included without any specific in 25 studies (62.5 %), that in 7 studies (17.5 %) the morphine equivalent daily dose was analyzed, in 3 studies (7.5 %) the treatment of each patient was described but the results do not differentiate between the various opioids and only 5 (12.5 %) of the studies focus on a single opioid (see detail in Annex 2: https://www.resed.es/Documentos/ArticulosImg/1022/07_rev_labori_usos_eng_anexo2.jpg).
The results of the reviewed articles are described below and classified in each of the four emerging thematic areas of matrix analysis: Epidemiology, risk factors, opinion and specific policies.
Epidemiology: Prevalence/incidence
The first studies performed in cancer patients detected a very low frequency of addiction to narcotic (opioid) analgesics, with figures of incidence of 0 % in follow-ups of 3 and 6 months (27) and of prevalence of less than 5 % in both adult and pediatric patients (28,29). In the same vein, a study reflecting the experience of 100 primary care physicians concluded that only 1% of these detected patients with opioid dependency, with incidence figures ranging from 1 to 2 patients per year (30). In contrast, a more recent study has reported a higher prevalence of opioid dependency, at 5.65%, although we must point out that in this case it was not specified whether opioids were medically prescribed or were illegal opioids (31).
In recent years, the term “inappropriate use of opioids” has been coined, which, as we referred to in the introduction, it includes various concepts other than addiction such as misuse, abuse, diversion, or chemical coping. Among the studies performed under this umbrella, the following studies should be highlighted: a prospective study concluding that 90 % of cancer patients comply with the prescribed opioid prescription (20), a retrospective study that detects only 0.1 % of opioid misuse in patients with cancer (32) and two multicenter studies aimed at assessing the safety of presentations of transmucosal fentanyl that report no cases of misuse (33,34).
In contrast, some studies report higher incidences of misuse and deepen the so-called aberrant behaviors. In this regard, the prospective study conducted by Kwon et al. (35), which detects 18 % of chemical coping in the consumption of opioids prescribed in cancer patients, should be highlighted. If we look at this, it is important to report that the figure of 18 % is the result of the assessment of the patient by a palliative care specialist, whereas when comparing with the general medical history of the same patients, in the latter case only 4 % is detected. This study provides interesting information about the possible under-diagnosis of the opioid misuse in cancer patients when they are not evaluated by a professional with broad knowledge of this subject. With regard to aberrant behaviors reflecting inappropriate use, two studies analyzed the type of opioid storage at home and the style of opioid use. These publications report alarming results, as they determine that more than 85 % of cancer patients unsafely store opioids and that between 13 and 26 % of patients use them in an unsafe manner, defined as sharing or losing medication (36,37). For adolescent and young adult cancer patients, two studies place the incidence of opioid misuse at about 12 %, with the most frequent aberrant behaviors being the concern of a third person for opioid abuse or for the management of the patient’s drugs and the way the patient names or talks about the drug: if the patient use the trade name or a slang term (38,39).
Focusing on systematic reviews, only two of them include information on the epidemiology of opioid misuse in cancer patients: the first focuses on opioid addiction in patients with chronic pain (22) and the second on the development of opioid dependence as analgesics (23). Hojsted and Sjogren (22) consider 5 studies in the oncology population (40-44) and describe a frequency of “addiction” ranging from 0 to 7.7 %. The second of the systematic reviews (23) includes two studies that look at cancer patients (45,46) but does not provide conclusive information, especially due to methodological difficulties. In these two studies considered by Minozzi, it is interesting to comment on the comparison made by Passik et al (45), which shows a low frequency of “misuse” of opioids in cancer patients, when compared with AIDS patients, where it is 6 times higher.
Risk factors and assessment tools
A total of sixteen studies (28,35-39,47-56) provide information on risk factors for opioid misuse. Age-related factors have been identified, with patients younger than 55 at higher risk (35,49,50). Depression (39,49,51,53,56), personal or family history of alcohol abuse (50,51,53), use of illegal substances (50,56), and severe pain (35,49,56) have also been described as risk factors. In addition, risk factors related to opioids themselves have been described, including the presence of severe withdrawal symptoms at the onset of treatment (28), the use of rescue medication only (47) and the prescription of high doses (47,49). In relation to this last point, it has been seen that the risk of opioid misuse increases the higher the prescribed opioid dose; although a specific dose from which this risk is higher is not defined, studies seem to point out that from an oral morphine equivalent daily dose of 50 mg the risk is higher. Finally, Silvestre et al. describe other risk factors such as the work situation, the patient’s perception of over-prescription, and the lack of awareness about drug return programs (37).
As mentioned in the introduction, various tools such as CAGE, SOAPP or ORT have been used to identify risk factors linked to the opioid misuse. In addition, studies using early detection techniques, such as the detection of opioids in urine, have been performed. Below are described the key data in this regard in detail.
Several studies assess the risk of inappropriate opioid use in cancer patients by linking it to the CAGE and CAGE-AID questionnaires (35,36,52-55). We can summarize from the results of the studies analyzed that the cancer patients who smoke have a higher percentage of positive in the CAGE questionnaire than the non-smokers (55) and that a positive result in this tool in patients with cancer has been shown to be a powerful predictor of chemical coping (35), unsafe use of opioids (36) and in addition increases the likelihood that the health care provider will request a urine test (54). It is important to note that, interestingly and apparently counterintuitively, CAGE-positive is a predictor of safe opioid storage (36).
Two studies performed a risk assessment using the ORT (50,51) and five studies evaluated the risk using the SOAPP test, two of which used the original revised version: SOAPP-R (38,56) and three of them the short version: SOAPP-SF (48,49,52). Note that two of these publications add a urine opioid screening test to risk estimation tools (50,56). In addition, one study evaluated the risk in 94 adolescent and young adult patients using a tool called “Screen for Opioid-Associated Aberrant Behavior Risk (SOABR) (39). Finally, a retrospective study analyzes the reasons for requesting an opioid urine test (54), being the following: Age under 45 years, African American race, low educational level, early stage of the disease, positive CAGE-AID, high pain score, low asthenia score. The studies performed with the ORT test determine a moderate-high risk of developing an opioid misuse in 43 % of cancer patients (50,51), while a more recent study using SOAPP-R refers a 32 % of misuse (56). Moreover, a SOAPP-SF study in a population that includes 91 % of cancer patients determines a lower risk, about 8 % of cases (52). Interestingly, patients classified as moderate-high risk by ORT show a high probability of presenting an abnormal urine opioid test, being described in 62.5 % of the patients studied, a fact that gives it a powerful role as a predictor (50). As described with CAGE, smokers have a higher risk of opioid abuse estimated by SOAPP-SF, and have greater mood swings, a higher possibility of using non-prescribed drugs, having a history of legal problems or use of illegal drugs (48). With regard to adolescent and young adult cancer patient, the risk-assessment study using SOAPP-R found that 39.5 % of patients were at high risk, and it is important to note that in this group, 33 % developed aberrant behavior at follow-up (38). In the study using the SOABR tool, risk factors for misuse were detected in 90.9 % of cases; personal and family history of mental disorder and the simultaneous use of more than one opioid the most frequent risk factors (39).
Opinion of professionals and patients
Fourteen studies on the opinion of professionals and patients on opioids or some aspect related to their misuse have been found: four of them focus on professional opinion (30,53,57,58), eight studies focus on cancer patients (29,59-65) and two studies on people in the general population (66,67).
Regarding the opinion of the professionals, a study by Vainio et al concluded in the 1980s that 50 % of primary care physicians reported never detecting patients with opioid dependence and 23 % did not use to prescribe opioids (30). In the same line, it was described that among professionals in oncology hospitals, 61 % of physicians were reluctant to prescribe opioids and 38 % of nurses to administer them (57). Later studies show a greater awareness of the topic by professionals, noting that primary care physicians place the prevalence of opioid dependence in cancer patients at around 10 % (58) and that palliative care and pain specialists show a 92 % consensus on the definition and detection of some specific aspects of opioid misuse as chemical coping with emotional distress (53).
In relation to what the cancer patients themselves think about, 52 % consider that they can easily present addiction to opioids (60) and 55.6 % express concern for presenting addiction (59), this concern being one of the most frequent barriers in pain management (63). Some patients relate morphine use to end-of-life, which may also make difficult the use opioids for pain management (62). With regard to breakthrough pain, 81 % of the patients asked in a study by Davies et al. report difficulty in taking rescue opioid because of concerns about the possibility of tolerance, overdose or addiction (61). In addition, there may be some cross-cultural difference in patient perception, as studies conducted in different countries provide different results. Thus, a study in Egypt concludes that 50.6 % of patients would reject tramadol due to fear of addiction (64) and another study conducted in Brazil found that 19.2 % of patients would refuse treatment with morphine because of fear of developing dependence (65). However, in a study of Swedish pediatric patients, no child or parent refused treatment with morphine for pain because of fear of addiction (29).
For the general population, two studies reported concerns about the risk of opioid addiction in the treatment of cancer pain (66,67).
Health center policies, programs and protocols on the opioid misuse
Two articles refer to health center policy on the opioid misuse (68,69). The first concludes that most palliative care centers included in the study do not perceive substance abuse or diversion as a problem, do not have established protocols, and do not train in this regard (68). The second study aims to measure the results of implementing an educational program aimed at improving the use and storage of opioids in cancer patients. Interestingly, such implementation improves aberrant behavior by reducing the unsafe use and storage of opioids in these patients (69).
DISCUSSION
The objective of the present review is to update available evidence regarding the opioid misuse in patients with cancer pain. The reviewed studies are very heterogeneous in methodology and results, but they can be grouped in four major thematic areas: Epidemiology, risk factors, patient and professional opinion, and specific policies. A brief discussion and analysis of the main results we have found in each of them can be found below.
With regard to the prevalence of opioid misuse or aberrant behavior, the results obtained are very heterogeneous in both adult and adolescent populations, with frequencies ranging from 0 to 26 % of misuse (32,37) and from 12 to 85 % of aberrant behavior (36,39). In our view, low prevalence figures, around 0 %, could be influenced by low detection of opioid misuse, which, according to results from Kwon et al., it could be related to the degree of specialization and awareness of the healthcare personnel in charge of the patient (35). The low detection of substance-related problems in patients with a medical condition is not a new topic, and in recent decades there are many studies on this regard. As an example, we could mention the ALCHIMIE study, which reported only 50 % of detection of harmful alcohol consumption in patients admitted to Internal Medicine units in 43 European hospitals (70).
The results reported for risk factors are more homogeneous, with a higher probability of having inadequate opioid use in young patients with a psychiatric history or a history of substance use, with a high intensity of pain and with use of high doses of opioids and/or rescue medication only (35,39,47,49,50,51,53,56). These characteristics allow us to stablish profiles of patients at higher risk of opioid misuse, a critical step in designing specific intervention protocols aimed at preventing and early detection of potential aberrant behavior. Some authors have attempted to quantify the risk through various tools, among which CAGE, ORT or SOAPP are the most frequently used. These studies offer a wide variability of results, being determined between 8 and 43 % of patients with moderate-high risk depending on the tool used (50,56). Because of the inconsistent results, it would probably be advisable to reach an international consensus to determine the best risk detection methodology and thus be able to conduct global detection and intervention policies.
In relation to the opinion of professionals, the overall impression is that healthcare workers are now more sensitive to this problem. In the first studies conducted, the results may seem contradictory since despite the perception of few cases of opioid dependence by professionals, they showed greater difficulty in prescribing opioids (30,57). More recent studies reveal greater concern about the opioid misuse in cancer patients by professionals (30,58) and international consensus has been reached on the definition of chemical coping of the use of opioids as the use of opioids to address the emotional distress characterized by inadequate and/or excessive use of opioid (53).
Regarding patient perception, it is important to emphasize that the concern regarding opioid addiction in half of cancer patients (59,60) and the association of morphine use with end-of-life (62) significantly interferes with pain management. In addition, differences in results according to the country in which the study was conducted are also noticeable.
With regard to the existence of specific policies in health centers, and despite the impression of increased awareness, attention is drawn to the absence of protocols and specialized training on this problem in palliative care centers (68). Interestingly, De La Cruz et al (69) show a marked decrease in the use and unsafe storage of opioids in cancer patients following the implementation of an educational program for patients. We therefore consider it of great importance that specific intervention programs for the training of professionals and patient health education can be designed and implemented.
CONCLUSIONS
Given the results of the present review, we consider that it is important to follow up carefully and in a structured manner any cancer patient to which opioid treatment is initiated and, more strictly, in those profiles described as having the greatest risk. Such longitudinal management would be in line with the transition in the palliative care model that has occurred in recent decades and which advocates early palliative care (71,72). In this regard, we believe that it is essential to establish protocols and policies for early detection and intervention against opioid misuse.
STRENGTHS AND LIMITATIONS
A potential limitation of this review is the heterogeneity of the studies included in terms of objectives, methodology and quality. Nevertheless, the integrative systematic review methodology has shown to minimize the risk of ignoring relevant information (73).
The main strength of this inclusive review is the comprehensive search and inclusion of a selection of relevant publications on the subject. In addition, this methodology has enabled us to synthesize information from a wide range of sources, we believe that this contributes to a better understanding by studying phenomena from a wide range of points of view. Although the number of publications about the opioid misuse in cancer patients is not very high, this review updates current knowledge and provides clinicians and researchers with a valuable reference to continue working to improve patient care. In this sense, the results of the systematic review may represent a strong starting point for designing early detection and intervention protocols in this population and for conducting new research studies providing greater evidence about the profiles of patients at risk and successful intervention strategies.
CONFLICT OF INTEREST
The authors of the present study have no conflict of interest to declare.
REFERENCES