DOI: 10.20986/resed.2019.3681/2018
ORIGINAL
Rapid opioid detoxification
M. J. Pujante Tortosa1 , M. C. Ribera Montés1 , C. Embid Román1 , F. Pascual Pastor2 y C. Sánchez Pérez3
1Médico Adjunto Servicio de Anestesiología. Cuidados Intensivos y Unidad de Dolor. Hospital General Universitario de Elda. 2Coordinador de Unidad de Conductas Adictivas. UCA Departamento de Salud de Alcoi. 3Jefe del Servicio de Anestesiología, Cuidados Intensivos y Unidad de Dolor Hospital General Universitario de Elda. Alicante, España
Received: 26-04-2018
Accepted: 14-11-2018
Correspondence: Calixto Andrés Sánchez Pérez
sanchez_cal@gva.es
ABSTRACT
Introduction: For the last 15 years we have witnessed a steady increase in opioid consumption. Being aware of an undertreatment in certain pain situations, many health care providers have encouraged their physicians to prescribe opioids to avoid unnecessary suffering. Such encouragement, also by means of switching from the traditional paper prescription to the current electronic one, has led to a wide spread in opioid prescription even among those medical specialities which never did before. Besides, new synthetic opioids with apparently less side effects, favourable kinetics and easer to take, might have arosen a wrong impression of unreal harmlessness. Therefore, the increased prescription and its obvious consequence of consumption has led to an alarming increase in the number of side effects, proving our patients not to be so well controlled. We have perceived in our Health Department several different patients with opioid consumption abuse derived from medical prescription with potential life threatening side effects, that´s why we have conducted a medical path for their detoxification.
Aim: To perform a safe fast opioid detoxification (FOD) in our fully monitored patients.
Method and materials: To perform our FOD path we previously admit the patients in our ICU unit. After a careful clinical, psychological, social and biological assessment, and having requested their informed consent, we monitor all their vital constants in bed and we start a deep polymodal sedation up to the required level for each patient, getting even ready for oral intubation and mechanical ventilation if needed. Our regular vital maintenance is based on fluids, deep vein thrombosis prophylaxis, digestive prophylaxis, physiotherapy, urine output and blood tests for 96 hours. Having achieved our goal, regarding the patients are stable, they are discharged to the ward for an additional 48 hours period, with psyquiatric treatment and under the care of the Addictive Conducts Unit. The patients are finally discharged from hospital with a multimodal supervision and treatment conducted by our Pain Unit, Addictive Conducts Unit and Physical Rehabilitation.
Results: We describe the results achieved with two different drug approaches which combine different pharmacological groups frequently used for detoxification: midazolam, propofol, ketamine, clonidine and naloxone, for our aim of succeeding in keeping the patients opioid-free without endangering their haemodynamic, breathe or biology.
Conclusions: FOD has proved to be a successful treatment in rescuing the patients from a living hell out of which they would have found it impossible to leave without qualified help. We deem it safe with the right ICU surveillance, since no major complications have occurred, but a thereafter following and help is mandatory, since, like any other patient attended at a Pain Clinic, they require a favouring social and familiar environment to avoid any relapse. Finally, and given our results, we consider this detoxification method right and safe but highly costly in resources.
Key words: Opioids, opioids abuse, fast opioid detoxification, FOD.
RESUMEN
Introducción: En estos últimos 15 años, el incremento del uso de analgésicos opioides ha sido progresivo y elevado. La percepción de la existencia de cuadros de dolor mal tratados ha provocado que muchos sistemas de salud incentiven a los profesionales en el uso de opioides, para evitar episodios de sufrimiento inútiles y estériles. Dicha incentivación, el uso de receta electrónica, la facilitación y simpleza de prescripción tras la desaparición de receta de estupefacientes tradicional, supuso una liberalización significativa y uso de opioides progresivo entre todas las especialidades médicas que habitualmente no los prescribían. Además, la aparición de nuevos opioides sintéticos, con aparentemente menores efectos secundarios, fáciles de usar y con buen perfil farmacocinético, quizás ha suscitado una confianza irreal en la inocuidad de dichos fármacos.
Estos últimos años han sido años de alta prescripción y de hallazgo de efectos no deseados por los elevados consumos y prescripciones un tanto quizá alejadas de la idoneidad y poco control sobre los pacientes. Nosotros detectamos en nuestro departamento de salud varios casos de pacientes con problemas reales derivados del consumo de opioides, de origen iatrogénico, siempre por prescripción médica legal y con gravísimos efectos secundarios, que conllevaban riesgo vital.
Objetivo: Realizar una deshabituación rápida del consumo de opioides, sin poner en riesgo la salud y vida del paciente, de un modo rigurosamente monitorizado y controlado.
Material y métodos: Nuestra comunicación científica se basa en la descripción del trabajo realizado sobre un grupo de pacientes afectados por un elevado consumo de opioides. Nuestro método de deshabituación a los opioides consiste en el ingreso de los pacientes en la Unidad de Cuidados Intensivos del Servicio de Anestesiología de nuestro hospital para la retirada absoluta de los mismos. Se realiza en primer lugar una valoración basal clínica, psicológica, social y biológica, y tras solicitar un consentimiento informado, se procede a una retirada de los opioides, con monitorización avanzada e inicio de sedación profunda multimodal, hasta el nivel que sea necesario para cada paciente, incluso con previsión de posible intubación orotraqueal y asistencia ventilatoria. Realizamos mantenimiento vital convencional de cuidados intensivos, con fluidoterapia, profilaxis antitrombótica, protección digestiva, fisioterapia, control de diuresis y control bioquímico, metabólico y nutricional durante 96 horas. Posteriormente, el paciente, tras asegurar su estabilidad y seguridad, pasa a una planta de hospitalización convencional durante unas 48 horas, con tratamiento de perfil psiquiátrico manejado por la Unidad de Conductas Adictivas. Tras ser dado de alta hospitalaria, se continúa tratamiento y control por Unidad de Conductas Adictivas, Unidad de Dolor y Unidad de Rehabilitación Física.
Resultados: Describimos los resultados obtenidos con el uso de dos pautas en las que se combinan distintos grupos farmacológicos usados para la deshabituación: midazolam, propofol, ketamina, dexmedetomidina, clonidina y naloxona, en la consecución del mantenimiento del paciente libre de opioides garantizando la estabilidad hemodinámica, respiratoria y la seguridad biológica del paciente.
Conclusiones: La desconexión rápida de opioides es un tratamiento eficaz, que recupera al paciente de un infierno vital grave del que difícilmente se puede salir sin una ayuda externa. Lo consideramos un método seguro, ya que no se nos ha presentado ninguna complicación severa, aunque son pacientes que precisan de unos cuidados médicos de vigilancia intensiva. El posterior seguimiento y ayuda es imprescindible, aunque como pacientes de dolor, precisan de un buen entorno social y familiar, para conseguir el apoyo necesario y no volver a recaer. Por todo ello, y en base a los resultados obtenidos en nuestro estudio, consideramos que es un método adecuado y eficaz, aunque caro en recursos.
Palabras clave: Opioides, abuso de opioides, deshabituación rápida de opioides, DRAO.
INTRODUCTION
The use of long-term opioid therapy for non-cancer chronic pain (NCCP) has increased significantly in recent decades, as well as the subsequent introduction of rapid-release opioid formulations in these cases could have produced an increase in cases of opioid addiction in patients with NCCP (1). The proportion of patients with iatrogenic addiction to opioids is very difficult to estimate due to the absence of definitions and specific criteria for patients with chronic pain and opioid use (2).
The criteria used for the diagnosis of opioid consumption disorder (OCT) are based on the Diagnostic and Statistical Manual of Mental Disorders (DSM), 5th edition (DSM-5) (3).
The DSM-5 defines the opioid use disorder (OUD) as a set of cognitive, behavioral and psychological symptoms that lead an individual to the continued use of a substance despite the problems related to its use (4).
The abuse of opioids produces alterations in brain circuits, which are the underlying causes for the development of dependency and addiction to these substances. Dependence refers to the imperative need to continue the use of opioids to avoid withdrawal syndrome, and addiction is defined as a chronic and recurrent disease of the brain characterized by the pursuit and compulsive use of substances despite their harmful consequences, inability to stop using the substance, neglecting work, social or family obligations and sometimes, depending on the substance, tolerance and deprivation or withdrawal.
Risk factors associated with an increase in the inappropriate use of opioid analgesics when prescribed for the management of chronic pain, such as substance abuse disorder, family history of substance abuse, associated mental illness, history of legal problems or jail sentences, white race and under 40-45 years of age, have been described (5).
In the pain units, patients referred by other specialties are treated. These patients are diagnosed of various pathologies, with chronic pain in general valued from moderate to severe, which in its evolution require the use of opioids. These treatments can potentially cause addition symptoms. Therefore, an exhaustive follow-up to detect the cases that could develop such symptoms is required. This follow-up should be conducted by the professionals specialized in the treatment of pain.
Other important concepts to consider are tolerance and dependence. Tolerance is the state of adaptation in which an increase in the doses of the opioid is required to obtain the desired effect, or there is a decrease in the effect of the substance over time (6).
The continued use of opioids for pain management creates dependence. An alteration in the physiological response resulting from the adaptation of the opioid-receptor binding due to chronic use occur in the physical dependence on opioids. This chronic use is characterized by the presence of OWS occurring after abrupt cessation, rapid reduction, decrease in level of the drug in blood and/or the administration of an antagonist (7). It is important to differentiate this term of addiction, which describes a chronic neurobiological disorder that involves an aberrant use of the opioid and a maladaptive social behavior that implies a loss of self-control that leads to compulsive and often self-destructive use (8).
In the present article we describe six cases of rapid opioid detoxification (ROD) conducted in the recovery unit of our hospital, between 2011 and 2016, of patients showing symptoms of opioids addiction.
ROD: DETOXIFICATION METHOD
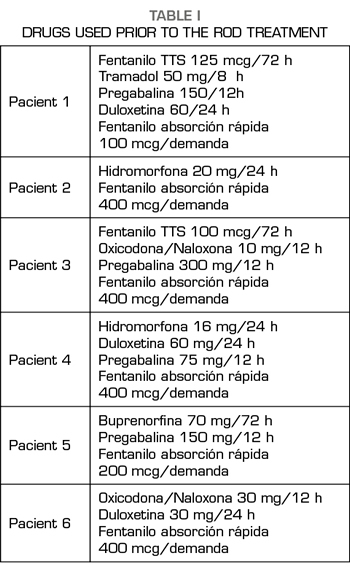
All the patients had been diagnosed with neuropathic pain, of years of evolution, with values > 7 regarding pain intensity in the visual analogue scale (VAS), in which different neuromodulators and opioids had been used from lower to higher, as indicated the World Health Organization (WHO) analgesic ladder (Table I).

They are two women and four men aged 36-53 years (in the year in which the ROD was performed) socially integrated with relatives who supported them, and without medical history that contraindicated the detoxification.
All the cases described in the present study were diagnosed in the consultation of the Pain Unit at our Health Department (220,000 inhabitants). Four of them referred from primary care and after being treated in our unit and two of them after referral from primary care with treatments already established by their general practitioners.
All of them had a point in common, which consisted in the use of fast-acting fentanyl for the treatment of non-cancer breakthrough pain some time before they began to increase their care demands for its prescription, since its consumption was increasing not only regarding the daily doses administered but also regarding the amount (micrograms) prescribed. This fact, together with the appearance of agitation and aggressiveness in the consultations and the denial that their baseline treatment was effective, verified by the anesthesiologists, by our nurse who answers telephone consultations in the PTU, as well as by the relatives, were the symptoms that led us to diagnose the addiction syndrome and to suggest a solution (4).
After the diagnosis of addiction syndrome (DSM-5 level 3, severe OUD), an appointment was booked for each patient that should be accompanied by a close relative. They were thoroughly informed about the addiction the patient suffered and the possibility of entering the recovery unit, where patients would be treated for a few days, the drugs that they would be using, the monitoring of vital signs and the risks. All 6 patients accepted and signed an informed consent together with their family member and an anesthesiologist from our unit.
METHOD
All the patients were admitted in a fasted state in the morning of a Monday. A peripheral vein was catheterized, a nasogastric (NG) tube and a urinary catheter were placed and blood was collected for a baseline analysis consisting of a blood count, biochemistry and coagulation determinations.
Non-invasive blood pressure, ECG, O2 saturation, diuresis and hourly temperature were monitored.
Two treatment groups were established (Table II).
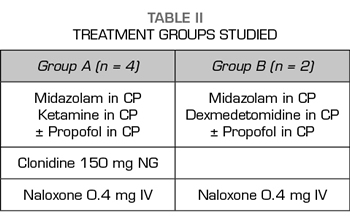
In four of the patients, the basic treatment consisted of an infusion of ketamine and midazolam plus propofol, which was reserved as a drug to be used, if after hours of admission without the administration of any opioid the patient needed a deeper sedation. These four patients were administered 150 mg 1 tablet NG clonidine every 12h, SC enoxaparin every 24h at prophylactic doses for deep vein thrombosis (DVT) and 40 mg IV omeprazole every 24 hours. For the symptoms and signs expected in opioid withdrawal (Table III) (such as hypertension, diarrhea, muscle-ages, vomiting, anxiety, etc.), we protocolized the administration of labetalol, loperamide, paracetamol/desketoprofen and ondansetron, depending on the appearance of those symptoms and signs, as long as there were no individual contraindications for their use.
Dexmedetomidine and midazolam were used for sedation during the detoxification process of the other two patients. Similarly, to the first four patients, propofol was used to increase the level of sedation only when the signs of withdrawal began. Furthermore, patients also underwent gastric protection, DVT prophylaxis with low molecular weight heparin due to prolonged immobilization, but in the latter two cases clonidine was not used orally, because clonidine is a molecule acting at the central nervous system on the same receptors as dexmedetomidine (9).
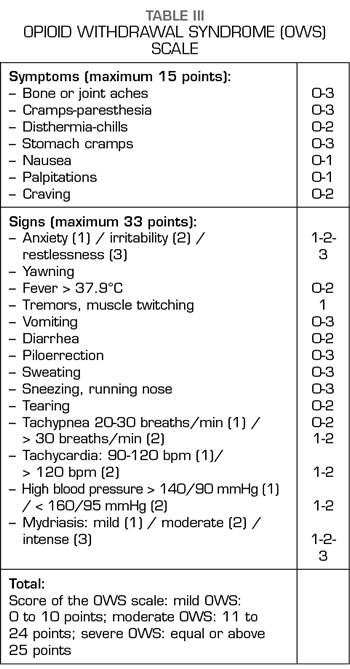
All patients, during the process, were assessed using the rating scale of the opioids withdrawal syndrome (OWS) until signs of detoxification ceased (Table III) (10).
RESULTS
Doses used were always individualized according to the needs of each patient and in increasing doses, according to their needs:
– Midazolam: perfusion started from 0.05 to 0.1 mg/kg/h, reaching maximum doses of 7 mg/h.
– Ketamine: started at 0.1 mg/kg/h and reached maximum doses of 20 mg/h.
– Dexmedetomidine: started at 0.2 μg/kg/h and reached doses of 1.4 μg /kg/h.
– Propofol: started as needed by the patients and their doses ranged from 1 mg/kg/h to 2 mg/kg/h.
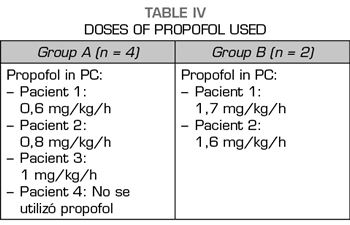
The two patients treated with midazolam and dexmedetomidine required perfusion of propofol between 10 and 12 hours after the beginning of the detoxification process; while only one case out of the 4 patients treated with midazolam, ketamine and clonidine did not require perfusion of propofol. Perfusion of propofol began later, between 30 and 36 h after the beginning of the process of detoxification, in the other 3 patients.
In the 4 cases in which the sedation consisted of an infusion of midazolam and ketamine, the signs of the OWS were less marked than in the two cases in which we used infusions of midazolam and dexmedetomidine. In addition, the perfusion doses of propofol in the first 4 cases were lower than in those patients of the midazolam-dexmedetomidine group (Table IV).
Both ketamine and dexmedetomidine infusions were not maintained for more than 72 hours, and they were always progressively reduced until discontinuation, maintaining the perfusion of midazolam and propofol, which subsequently decreased gradually until discontinuation.
Regarding the cessation of symptoms and signs of the OWS, these occurred between 72 and 96 hours in all the cases studied. Then a dose of 0.4 mg IV naloxone was administered and the responses of each patient were observed, without any of the cases showing signs of opioid deprivation.
The length of the stay at the recovery unit was five days for all patients, except for one case who stayed at the unit for 8 days. In the latter case, the patient was also addicted to nicotine and with a history of moderate associated alcoholism. There was no case of respiratory depression or severe pain or psychomotor agitation that required intubation of the patient.
The patients are usually discharged on the fifth day to the conventional hospitalization ward, where they stayed for 48 hours undergoing alternative treatment, and monitored in case any type of complication occurs.
After discharge, treatments with neuromodulators, olanzapine and in two cases also with THC (tetrahydrocannabinol) in sublingual spray for crisis of neuropathic pain began, in case.
All the patients, after the detoxification, were referred to the unit of addiction behavior for following-up the progress and continued with the periodic checkup in our unit.
DISCUSSION
Opioid addiction is a complex disease that is difficult to treat. The treatment is divided into three processes: stabilization, detoxification and maintenance therapy (11). Stabilization consists in the substitution of the opioid for treatments that ensures that the use of the drug is independent of the mental state and the circumstances. Detoxification consists in the withdrawal of the opioid safely and effectively, minimizing the withdrawal syndrome (with opioid receptor agonist drugs, such as methadone or buprenorphine or with agonists of alpha-2 adrenergic receptors such as clonidine or dexmedetomidine). Maintenance therapy consists of relapse prevention (usually with the administration of opioid receptor antagonists, such as naloxone or naltrexone).
The problems that clinical experience has helped us to observe is that, since the introduction of treatments outside of the data sheet (non-cancer patients) of the fast-acting fentanyls, the “fascination” that produces the effect of the drug through areas of brain stimulation (with great feeling of gratification) is greater than analgesia in at the beginning. This means that almost all of our non-cancer patients, included in the FOD program, tend to abandon the baseline treatment, opioids, adjuvants, etc., in favor of performing analgesia mediated by the rapid opioid exclusively and in doses absolutely larger than those recommended by their doctor, both in amount per dose and in frequency of them. In fact, this is the main important recommendation we make: a comprehensive pharmacotherapeutic follow-up of patients who are treated with fast-acting fentanyl out of the datasheet, as well as the performance of a pact of time and evaluation of therapeutic efficacy while evaluating other alternatives that would allow an early withdraw of these drugs. In general, we can say that the incidence of overdose and iatrogenic problems with this fast-acting fentanyl is almost non-existent when prescribed and controlled from our Pain Unit, but not when prescribed by other medical specialties, with less knowledge of the problem generated by the chronic use of opioids.
The traditional management in the 1970s of this syndrome implied either replacement by a long-acting opioid such as methadone and its subsequent gradual withdrawal, or the non-use of opioids (clonidine with adjuvants such as analgesics, hypnotics and benzodiazepines). Generally, in both processes, a mu-receptor antagonist such as naltrexone was gradually introduced (12,13). The rate of failure and relapse in both cases was high due to the discomfort and distress that the patients experienced during the procedure. Therefore, in the 1980s, Loimer et al. (14) developed for the first time this process of detoxification with general anesthesia and intubation, based on Yale University publications (15) on ROD methods.
From then, different researchers have been introducing improvements and modifications to the ultra-ROD technique (16).
Studies on the efficacy of gradual opioid withdrawal methods using methadone or buprenorphine treatment versus new methods of ROD and ultra-ROD have not been able to demonstrate the clear benefits of one technique or another. Clinicians should be guided by the response of patients to determine the duration of the opioid withdrawal period (17).
Johnson and Carr (18), proposed the following classification in 2003:
– In the ultra-ROD, the use of general anesthesia can be used for less than 6 hours.
– In the ROD, a deep sedation varying from 6 to 72 hours in the studies described.
Several studies have described rapid detoxification techniques over the years, all using potent anesthetic agents that induce deep hypnosis to mitigate the symptoms of the OWS. It is not known exactly by which mechanisms anesthetic agents are able to block the expression of opioid withdrawal, but one of the explanations could be in the interference of these drugs with glutamate, which is closely associated with a noradrenergic hyperactivity that in part is behind the pathophysiology of the withdrawal syndrome (19).
The efficacy results for ultra-ROD are not clear and, therefore, recommendations on their use are controversial. In a recent review published in 2015, ultra-ROD techniques are not recommended under general anesthesia due to the registry of severe complications in the literature, including cardiac arrest and death (20,21). In a systematic review of 5 randomized studies, they conclude that its use is not advisable due to the lack of benefit and the potential documented risks and the high costs that generate the admission of patients in intensive care units based on general anesthesia or deep sedation (22).
Another point to discuss is the different drugs that we have used in the development of our ROD protocol.
In opioid detoxification techniques through induction of general anesthesia, the use of drugs such as clonidine and dexmedetomidine is approved, drugs that are used in a protocolized manner in our intensive care unit. We describe below the pharmacological reasons for which these drugs are effective, although their indication is out of the data sheet.
Due to the high noradrenergic activity that is triggered after the withdrawal of opioids (characteristic withdrawal syndrome), the use of α2 agonists, such as clonidine or dexmedetomidine, has been successful in a large number of published studies. Their main limitation to use is the antihypertensive effect. Their beneficial effects in this task are their sedative activity, the reduction of the activation of the sympathetic nervous system and the decrease in the requirements of opioids described by mechanisms poorly known but that seem to be related to their activity on the nucleus ceruleus.
The α2 agonist drugs are usually combined with another type of drugs, which we also use in a protocolized way in our study. These drugs are benzodiazepines, in our case continuous perfusion (CP) of midazolam, ideal for reducing levels of anxiety and improvement of sedation levels, ondansetron for the reduction of nausea and vomiting and loperamide for the treatment of diarrhea in patients with withdrawal syndrome. Other drugs used in our study are propofol, an hypnotic drug that in CP controlled by Target Control Infusion (TCI) is ideal for sedation to maintain spontaneous ventilation, and ketamine, whose sympathetic activity could be conflicting in its use for opioids detoxification but, as we observed in our study, ketamine is useful in combination with the rest of the drugs used due to its analgesic potency.
Ketamine is a drug widely used in anesthesia. Ketamine is classified as an NMDA receptor antagonist but has many other actions on mu, delta and kappa opioid receptors, and also Ketamine inhibits the reuptake of serotonin, dopamine and norepinephrine (23). This mechanism of action has been used in anesthesia to decrease tolerance to opioids, reduce the consumption of analgesics and increase the time in which patients begin to consume analgesics in the postoperative period (24). Moreover, the use of ketamine makes sense in patients who have established chronic pain.
Previous studies have described a successful opioid detoxification with ketamine, even with ketamine orally (23). In a group of 58 patients, the administration of continuous ketamine infusions in subanesthetic doses, 0.5 mg/kg/h dose, was also successful. The group treated with ketamine experienced better control of OWS (25). These data are consistent with the findings observed in our study that, despite the limitations of the sample size, the group treated with ketamine presented fewer signs and symptoms associated with the withdrawal syndrome due to opioid deprivation and lower use of propofol.
Our medium- and long-term results on the treated patients reaffirm us in the need to make the patient see:
1. The continued risk for relapse that patient will have throughout life.
2. That patient must inform the anesthesiologist immediately if he/she is going to have surgery.
3. That patient will be monitored frequently by specialists in pain, addictive behaviors, psychiatry and psychology, given that patient has overcome a critical illness and the health of the patient should be monitored.
In general, our patients report that they have got a “second chance” at life. They continue with symptoms of pain but, in general, they refer less intense pain than prior the treatment, but above all they report having gained infinitely in quality of life, since they were immersed in the spiral of side effects of opioids at all levels, worsening even the painful perception severely. We believe that these patients will need analgesic/adjuvant treatment, perhaps for life, but our limitation in the number of patients and the time after treatment does not allow us to know the long-term development.
Unfortunately, we have had a therapeutic failure in a young patient. After an extraordinary initial success in this patient with an improvement in quality of life and absence of pain (which also encouraged her to want to create a support group for patients with similar conditions to prompt them to undergo ROD). After 6 months, this patient began to reject everything, being evaluated by our entire multidisciplinary care group without being able to overcome the relapse, she began to take opioids out of the prescription of our environment, moving away from the guided and recommended therapy for her.
CONCLUSIONS
– According to the duration of our opioid detoxification method, we could classify it within the group defined as ROD.
– In all cases, a good level of sedation has been maintained (measured by the Richmond Agitation Sedation Scale (RASS)) without requiring invasive assistance of the airway and maintaining spontaneous ventilation.
– No patient in the study had severe complications. Only a minor bronchoaspiration event occurred in one patient without clinical consequences.
– The amnesia of the patients of almost the entire length of the stay at our intensive care unit is noteworthy, even the period they were awake and collaborating with the nursing staff.
– The two cases that required the highest use of drugs were those that had the highest opioid use at baseline. These were treated in the group of dexmedetomidine and midazolam, without ketamine, a fact that should be noted for the analgesic and hypnotic effect of ketamine. In addition, patients in this line of treatment showed more symptoms and signs of OWS. These data are merely observational due to the small sample size of our study.
– Studies with larger sample size are needed to achieve conclusions with more quality and scientific evidence.
– We strongly recommend selecting patients very well to perform this technique. Thereby, they would be able to assimilate the effort and risk they are going to undergo, so that they become aware that a drastic change in their life is going to take place, and accept it.
– We sincerely believe that the iatrogenesis due to the poorly controlled use of opioids should be solved with more training on its use, and the early detection of patients susceptible to developing problems with these drugs.
– We think that this technique represents “a second chance” from which selected patients can benefit.
CONFLICTS OF INTEREST
Authors declare no conflicts of interest. No funding has been obtained to perform this study.
BIBLIOGRAPHY