DOI: 10.20986/resed.2021.3902/2021
REVIEW
BOTULINUM TOXIN IN THE TREATMENT OF MYOFASCIAL PAIN SYNDROME
TOXINA BOTULÍNICA EN EL TRATAMIENTO DEL SÍNDROME DE DOLOR MIOFASCIAL
D. C. Nájera Losada1
J. C. Pérez Moreno1
A. Mendiola de la Osa2
1Unidad del Dolor, Hospital Universitario Virgen de las Nieves. Granada, España
2Unidad del Dolor, Hospital Universitario Puerta de Hierro. Majadahonda, Madrid, España
ABSTRACT
Botulinum toxin injections have been used in pain treatment associated with pathologies such as focal dystonia, spasticity, headaches and myofascial pain. However, results from botulinum toxin trials in myofascial pain syndrome (MPS) are contradictory.
The objective of this paper is to analyze the evidence of botulinum toxin type A (BTA) efficacy compared to placebo in myofascial pain management. Literature search was performed in PubMed, Web of Science (WoS), Scielo and Scopus, using the following key words: myofascial pain, trigger point, botulinum toxin and botox. Eleven clinical trials comparing BTA versus normal saline solution (NSS) met the inclusion criteria. Although most of the clinical trials analyzed cannot demonstrate a BTA superiority, it would not be correct to conclude that botulinum toxin is not indicated in miofascial pain treatment due to the great heterogeneous patient selection, variability in BTA doses, different trigger points (TP) injections techniques, variability in trials duration, and absence of cost-effective analysis.
More specific clinical trials are required using homogeneous samples to provide conclusive evidence for BTA in the MPS treatment.
Key words: Myofascial pain, trigger point, botulinum toxin, botox
RESUMEN
Las infiltraciones con toxina botulínica han sido utilizadas en el tratamiento del dolor asociado a múltiples patologías, como distonías focales, espasticidad, cefaleas y dolor miofascial. Sin embargo, los resultados de los diferentes estudios realizados con toxina botulínica en el síndrome de dolor miofascial (SDM) son contradictorios. El objetivo de la presente revisión es analizar la evidencia de la eficacia de la toxina botulínica tipo A (TBA) frente a placebo en la disminución del dolor crónico de origen miofascial.
Se realizó una búsqueda bibliográfica en PubMed, Web of Science (WoS), Scielo y Scopus, utilizando las siguientes palabras clave: dolor miofascial, punto gatillo, toxina botulínica y bótox. Los estudios que cumplieron los criterios inclusión fueron once ensayos clínicos que comparaban la TBA frente a solución salina normal (SSN).
Aunque en la mayoría de los ensayos clínicos analizados no podemos evidenciar un beneficio de la TBA frente a SSN, no sería acertado concluir que la toxina botulínica no está indicada en el tratamiento de dolor asociado al SDM, dado que existe una selección de pacientes muy heterogénea, hay una gran variabilidad en la dosis de toxina botulínica, se usan diferentes técnicas de infiltración de los puntos gatillo (PG), la duración de los estudios es variable y no hay estudios que realicen un análisis costo-efectivo.
Se necesitan ensayos clínicos más específicos, con muestras más homogéneas, que nos permitan sacar conclusiones acerca del papel de la TBA en el tratamiento del SDM.
Palabras clave: Dolor miofascial, punto gatillo, toxina botulínica, bótox
Correspondence: Diana Carolina Nájera Losada
diananajeralosada@gmail.com
Received: February 15, 2021
Accepted: April 17, 2021
INTRODUCTION
Myofascial pain syndrome (MPS) is a very common musculoskeletal condition in the general population and underdiagnosed on several occasions. It is defined as a regional muscle pain associated with the presence of TP. In turn, these TP are described as a tense muscular band, hypersensitive, that when palpated, generates a referred pain and a muscular contraction (1,2).
MPS can be classified as a primary syndrome where there is no association with other conditions or secondary syndrome occurring in conjunction with other painful conditions such as whiplash syndrome, root pain, osteoarthritis, fibromyalgia, and fractures (3).
The exact etiology and pathophysiology of myofascial TP are still unknown. It has been suggested that its development is related to an excess release of acetylcholine, producing a sustained muscle contraction with the subsequent formation of a TP (1). This sustained muscle contraction leads to an increase in the concentration of nociceptive and inflammatory neurotransmitters within TP, making it a permanent nociceptive stimulus facilitating central sensitization, generating a chronic pain picture (1).
In the early stages of MPS, central sensitization can be reversed with pharmacological treatment (using NSAIDs, corticosteroids, tricyclic antidepressants, vasodilators, muscle relaxants) or by puncturing TP with local anesthetic (with or without corticosteroid), dry puncture, and physiotherapy. The results may be incomplete with persistent pain in TP because the long-term benefit of these treatments is transient. Botulinum toxin was chosen (out of the data sheet) looking for long-term treatment due to its long duration of action and its localized effect on TP itself, which theoretically could be effective by avoiding recurrences because it would cause the decrease of electrical activity at this level, inhibiting muscle contraction and preventing recurrence of the painful point. Botulinum toxin type A (BTA) has also been used in several cases of chronic pain associated with focal dystonia, spasticity, and headaches (1,4,5).
BTA has a fascinating history; it was described in Germany by Justinus Kerner in the 18th century (as a picture of what we now know as botulism) after an epidemic produced by the consumption of poisoned sausages (botulus in Latin means sausage). Kerner thought that there was a toxin affecting autonomic and motor nerve conduction that could be effective in hyperexcitability situations if used in low doses. Hypotheses about potential therapeutic uses of the toxin subsequently began, but it was not until 1977 that the Food and Drug Administration of the United States (FDA) authorized Alan B. Scott to study BTA in humans. Scott founded the company Oculinum that would produce the BTA, and this allowed the other researchers to conduct studies with this compound. Before the end of 1980, BTA was already widely used to treat strabismus, blepharospasm, dystonia, hemifacial spasm, and spasticity. At present, the indications of BTA have expanded exponentially, due in part to its mechanism of action because it is now known that BTA acts on multiple levels, as we will explain later (6).
BTA alters muscle contraction by preventing acetylcholine from being released at the neuromuscular junction. In the motor nerve ending, there is endocytosis of the molecule mediated by its heavy chain. Subsequently, the disulfide bridge between the heavy and light chains is broken, causing the release of the light chain to the cytosol. This will result in the rupture of the soluble NSF attachment protein receptors (SNAREs) that attach the synaptic vesicles to the cell membrane. Therefore, the binding and subsequent fusion of these vesicles within the membrane is avoided, preventing the release of neurotransmitters such as acetylcholine. This process takes two to three days to establish itself, and after this time, muscle weakness begins to appear. Clinical improvement is seen toward the third week, with a maximum effect observed two months after injection. Muscle weakness may last six months; however, the clinical effect lasts on average three months, which is the time it takes to regenerate nerve ending by creating new connections with the motor endplate (5).
In patients with dystonia, pain improvement has been seen before five days after the administration of the toxin or even after three months of its injection, indicating that there is a different analgesic effect than the one described above (5).
Although BTA inhibits the release of neurotransmitters in peripheral nociceptors, not all nerve cells exhibit receptors for the toxin, therefore, the effect at the level of sensory nerve endings is not as predictable as that observed in motor nerve endings (5).
In migraine, where there is sensitization of the trigeminal system, subcutaneously administered BTA decreases pain perception and intensity, secondary hyperalgesia, and blood flow in the affected area. In this case, the effect of BTA would be mediated by its action on C fibers and the TRPV1 receptor (5).
An additional effect of BTA has been described in hemifacial spasm because the toxin is taken with high avidity by the nerve endings of the muscles that show higher activity, such as those involved in involuntary movements (6).
In models of inflammatory pain induced by the administration of formalin in the rat’s leg, BTA has been found to reduce substance P and glutamate release. At the peripheral level, BTA decreases inflammation and local glutamate accumulation, improving pain rates in assessment scales in the rat. At the central level, BTA travels through the spinal cord, inhibiting the release of substance P from spinal neurons. In the model of ischemic pain secondary to sciatic nerve ligation in the rat, BTA injection into the affected leg has shown a reduction in the release of nociceptive interleukins and a compensatory increase in antinociceptive interleukins with the subsequent improvement in the pain behaviors of the animal. In in vitro studies, the application of BTA in cultured cells inhibits the release of the calcitonin gene-related peptide (CGRP), glutamate, and other pain mediators, very interesting data because they can be extended to humans. Another interesting effect of BTA has been described, causing inhibition of sodium channel function in sensory neurons and in the periphery, which can play a very important role in pain transmission. Recently, studies in animals and healthy volunteers suggest a central analgesic effect of BTA because they have shown improvement of pain in the two affected limbs with the unilateral application of the toxin, but further studies are needed to confirm these findings (7).
As a summary of the above, BTA could act on multiple levels, affecting pain transmission in the central and peripheral nervous system, decreasing behavioral manifestations secondary to pain through a wide variety of mechanisms; among these, its effect mediating nociception is almost as important as its effect at the level of the neuromuscular junction.
Furthermore, physical therapy has been shown to be beneficial in MPS. However, some patients have difficulty completing physiotherapy due to severe pain from a spasm refractory to conventional treatment. Therefore, a relaxation maintained with BTA could relieve pain in a prolonged manner, allowing patients to complete physical rehabilitation programs that eventually produce long-term pain relief (1).
However, the efficacy of BTA remains unknown due to the limited number of studies, size of samples used, and variability of the doses used for each TP (2,8).
Adverse effects of BTA are well documented and include: Excessive muscle weakness, weakness of the muscles adjacent to the infiltrated muscles, weakness of muscles in other body areas due to hematogenous spread, dry mouth (xerostomia), decreased sweating and ocular lubrication, rash, flu-like symptoms, brachial neuritis-like syndrome, ecchymosis, bleeding, and pain at the injection site (1). Most of the side effects obtained in studies with BTA in the MPS are related to flu-like symptoms and localized muscle weakness, which are transient and usually resolved within 7 to 10 days (1).
OBJECTIVE
The main objective of this review is to assess the efficacy of BTA versus NSS (placebo) in reducing chronic pain of myofascial origin.
STUDIES CONSIDERED IN THIS REVIEW
Only randomized, double-blind, controlled clinical trials were considered to use studies with low biases or confounding variables.
STUDIES THAT WERE NOT CONSIDERED IN THIS REVIEW
Clinical trials with a sample of less than 10 patients in any of the groups to compare, observational studies, clinical case studies, and in general any type of study that was not randomized. Studies comparing BTA with another type of injection that had a medication (e.g., local anesthetic or corticosteroid) were also not taken into account. Studies related to myofascial pain of craniofacial or pelvic origin were excluded from this review.
LITERATURE SEARCH
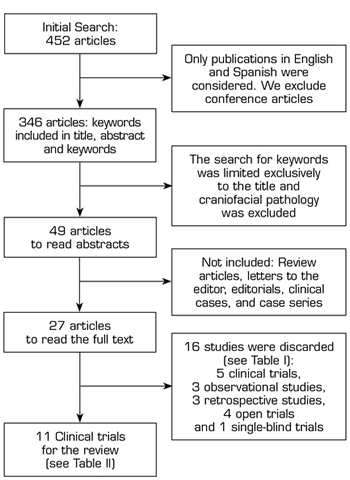
A literature search was performed in PubMed, Web of Science (WoS), SciELO, and Scopus, using the Boolean operators AND/OR and the following keywords: Myofascial pain, trigger point, botulinum toxin, and Botox. Initially, the search for these words was conducted in the title, abstract, and keywords, finding 452 articles (Figure 1). By excluding book chapters, conference communications, and limiting the search to human studies, presented in English or Spanish, published from January 2000 to May 2020, we found 346 articles. We obtained 65 articles by focusing the search of keywords on the titles of the articles found. After reading the titles of the articles, we found that there were several meta-analyses related to craniofacial myofascial pain (mainly associated with temporomandibular disorders or dysfunction), so studies related to this anatomical region were excluded from the present review; therefore, we obtained 49 articles; of these were excluded: review articles, letters to the editor, editorials, clinical cases and case series, finally leaving 27 articles to read in full text.

Fig. 1. Flowchart for the search of review studies.
A total of 16 out of the 27 articles (Table I) were discarded because they did not meet the inclusion criteria of this review. Five clinical trials were not included: The first clinical trial evaluated the effect of motor versus sensory stimulation associated with BTA in TP (9), the second clinical trial compared NSS versus local anesthetic with corticosteroid (10), the third clinical trial compared BTA alone or associated with lidocaine (11), the fourth clinical trial compared BTA versus bupivacaine (12), and the fifth clinical trial compared BTA versus methylprednisolone (13); 3 prospective observational studies (14-16), 3 retrospective studies (3,17,18), 4 open clinical trials (19-22) and 1 single-blind clinical trials (23).
Table I. Studies discarded from this review
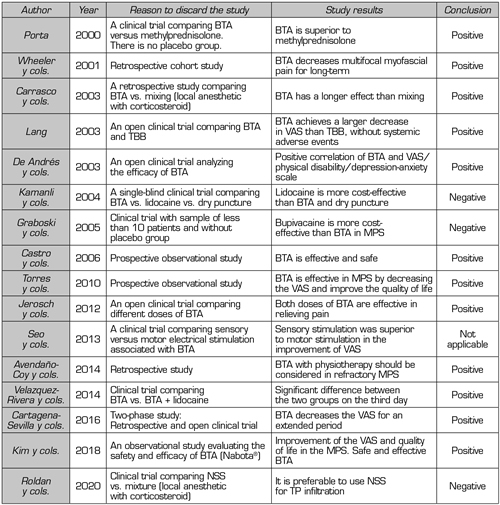
TP: Trigger point. MPS: Myofascial pain syndrome. VAS: Visual analog scale. BTA: Botulinum toxin type A. NSS: Normal saline solution.
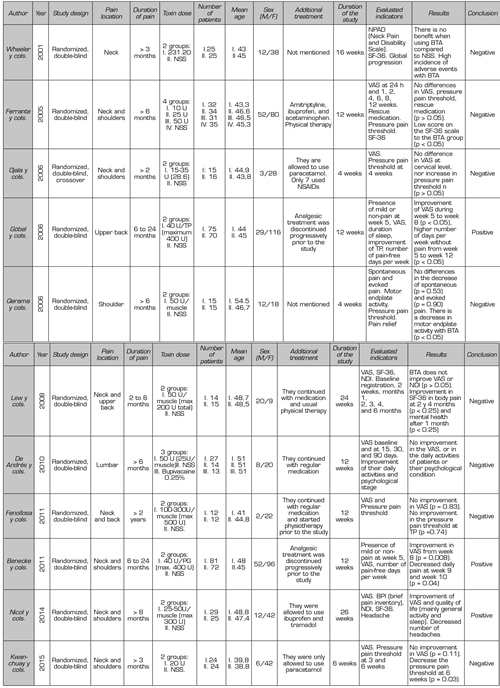
Finally, 11 clinical trials (Table II) comparing BTA versus NSS were considered for this review.
Table II. Clinical trials included in this review
ANALYSIS OF CLINICAL TRIALS INCLUDED
Although the quality of the clinical trials used for this review is very good (they score equal to or above four on the Jadad scale), their analysis is complex because many aspects vary in each study: Design, inclusion criteria, location of TPs, number and location of infiltrations, duration of the study, toxin dose, and method for measurement of results. In addition, not all report whether they maintained routine analgesia, initiation of physical therapy, rescue medication, and other complementary therapies throughout the studies, which poses a major challenge in drawing conclusions about the usefulness of BTA in MPS.
Ojala et al. (24) compared small doses of BTA (15 - 35 U) versus NSS; they infiltrated three to seven TP at trapezius, levator scapulae, and infraspinatus levels. The infiltrations were guided by electromyography (EMG). In this study, the authors report that using the algometer (to quantify the pressure pain threshold) is a reliable and objective measure, complemented with VAS. They found no difference in pain score or the pressure pain threshold in TP when using low doses of toxin in the neck muscles.
Wheeler et al. (25) They compared the efficacy of BTA versus NSS in TP of the neck muscles (mainly trapezius and lower neck part). They found no statistically significant differences in the improvement in the neck pain and disability scale (NPAD), quality of life (with the SF-36 survey), or overall patient assessment score. On the contrary, they found a high incidence of side effects, mainly weakness of the operated muscles. One unfavorable point this study could present is that most of the patients studied had a long-standing MPS (on average eight years), which can be difficult to control given the complexity of the already structured pain. The authors also emphasize the importance of combining BTA with physical therapy to optimize treatment and suggest that repeated sessions with low doses of BTA may be effective.
Lew et al. (26) compared the efficacy of BTA versus NSS at the level of the neck and upper-back muscles: trapezius, levator scapulae, head splenic, and other muscles in the posterior region of the neck. They infiltrated 50 U per TP, not exceeding the total dose of 200 U or 100 U on each side (no more than two muscles on each side were infiltrated). Their results found that BTA improved neck pain and disability, but when compared with the results of the control group (NSS), no statistically significant difference was found. They concluded that this result could be due to a placebo effect.
Göbel et al. (27) compared two groups (BTA vs. NSS). Their main objective was to assess the percentage of patients who had mild pain or no pain after five weeks of administering BTA in the upper-back muscles. Unlike other authors, they used higher maximum doses of BTA (40 U per TP, maximum ten TP). The results of his study were favorable: Given 400 U of BTA in ten TP, there was a significant improvement in VAS from the fifth to the eighth week, associated with the presence of more pain-free days per week until the twelfth week.
Ferrante et al. (28) compared the efficacy of three different doses of BTA (10, 25, and 50 U) versus NSS in TP infiltration. This is the only clinical trial that standardized a regimen of concomitant medical treatment (amitriptyline, ibuprofen, and paracetamol) associated with physical therapy. The authors found no differences between the BTA and NSS groups in pain reduction in VAS, pressure algometry, and rescue medication. In contrast with the Göbel et al. study, which infiltrated ten TP, this study did not include patients with more than five active TP at the neck and shoulder levels (maximum five TP could be infiltrated). These inclusion criteria could lead us to think that the severity of the MPS in these patients was lower, and considering that three analgesic drugs associated with physical therapy were administered concomitantly, we could see that a patient with a mild MPS (as presented in this case) would improve with conventional medical treatment without the need for interventional techniques; therefore, the results of this study should be carefully evaluated.
Qerama et al. (29) included patients with very specific pain in their study: Shoulder pain irradiating to the arm for more than six months, associated with a TP of the infraspinatus muscle. As negative points, patients having concomitant TP in other ipsilateral muscles (trapezius, supraspinatus) were excluded, and 12 patients (7 in the BTA group and 6 in the control group) had an associated diagnosis of complex regional pain syndrome. The authors concluded that BTA given in the TP of the infraspinatus muscle causes a significant decrease in motor plaque activity but has no effect on the intensity or threshold of pain.
Benecke et al. (30) used a protocol of ten TP to infiltrate in a standardized way in patients with neck and shoulder pain of myofascial origin, administering 40 U of BTA per TP. This study has the same methodology used as the study by Göbel et al., except for the standardization of the zones to be infiltrated. When comparing these two studies, the authors reported that administering BTA in individualized TP has an earlier and longer effect than the standardized TP regimen.
De Andrés et al. (31) evaluated myofascial pain at the lumbar level, selected quadratus lumborum muscle and iliopsoas muscle for ease of exploration, and for the referred pain pattern triggered by pressing TP. A positive point in this study is that they guided the puncture by fluoroscopy. They did not find that BTA decreases VAS, nor does it improve the daily activities or psychological status of the patients studied. They only found a decrease in post-infiltration VAS, and given the high cost of BTA; the authors considered that BTA should be used only in cases of pain refractory to other invasive techniques.
Nicol et al. (1) presented a study with a different methodology because they performed a test with BTA before conducting the clinical trial to determine which patients were going to have a positive response at six weeks. The authors assessed not only VAS but also the quality of life (SF-36 survey), disability (using the neck disability index: NDI), and headache (frequency and duration). The authors infiltrated the neck muscles, but not the stabilizing muscles of the scapula (infraspinatus, supraspinatus, and rhomboid) because of the possibility of worsening the symptoms by weakening these muscles. The dilution was 25 U/ml, and a maximum of 300 U was injected. The BTA was administered at half the thickness of the painful muscle, regardless of the location of the TP.
Their results showed a decrease in VAS, improvement in overall activity, and improvement in sleep after 26 weeks of BTA administration; they also found a decrease in the number of headache episodes per week. In contrast, patients receiving placebo had a worsening of pain and quality of life, which shows us the residual effect in patients who had received BTA in the first phase of this study, and that the differences observed in this second phase are not only due to a placebo effect.
Kwanchuay et al. (32) used small doses of BTA (20 U) and limited their study to the most painful TP at the trapezius level. They did not find that BTA reduced pain at six weeks compared to NSS. They considered VAS to be a subjective, uncertain, and inappropriate measure to assess musculoskeletal disease because it may be biased due to pain originating in adjacent muscles, without accurately assessing TP pain. On the contrary, they consider the algometer to be an instrument that gives objective and accurate measurements. They conclude that their study findings are positive because they demonstrated the effectiveness of BTA in MPS due to an increase in the pain threshold after toxin administration. The authors also emphasized trapezius stretches during study time.
Fenollosa et al. (33) found no statistically significant differences in the decrease in VAS or increase in the pressure pain threshold in TPs when comparing BTA versus placebo. These authors used an average dose of 300 U BTA, and all patients received physical therapy before and during the study. Although BTA was not statistically higher than placebo, for the authors, an improvement in the two-point VAS was clinically relevant in finding a 44.6% decrease in VAS in the BTA group versus 26% in the placebo group in the twelfth week. They conclude that BMT associated with rehabilitation therapy may be helpful in the treatment of patients with neck or dorsal MPS.
DISCUSSION
Following the main objective of the present review, we could state that there was no statistically significant improvement in VAS in patients receiving BTA versus NSS in the decrease of chronic pain of myofascial origin in eight of the eleven studies analyzed. This finding should be analyzed in depth because if viewed lightly, it could lead to the conclusion that BTA is not indicated in MPS. Before we continue with the negative studies for BTA, we would like to discuss the positive studies for BTA in MPS.
The same group of researchers performed two of the three studies showing an improvement in VAS with BTA. The studies had the same design and differed mainly by the technique followed to infiltrate the TP. In the study conducted by Göbel et al., the 10 most painful TP were infiltrated; and in the study conducted by Benecke et al., the 10 TP were standardized for all patients. This leads us to wonder what is the difference between the design of these studies and the other clinical trials. There are several points in the design that the other studies do not have: The first point is pain intensity because patients had moderate to severe pain, while other studies where BTA did not show a positive result (such as Qerama et al and Ferrante et al) had mild pain. The second point is that the number of TP patients should have to be included in the study (10 or more TP), which is directly related to the intensity of pain they suffered. The third point is the time of pain progression that patients had before the start of the study; they included patients with pain between six months and two years, while other studies (where there was no benefit from BTA) such as those by Wheeler et al. and Ojala et al. had on average pain progression times of 8.6 years and 10.5 years, respectively. Some authors considered that this time of progression is crucial because patients with pain of more than 1.5 years of progression have a worse response to BTA, possibly due to fibrotic changes in the affected muscle fibers (34,35). We believe that these three points could be important in assessing the positive results of BTA in the MPS.
The third positive study for BTA is that of Nicol et al., in which the methodology was totally different from that of the other published studies. Their protocol had two phases: In the first one, they used the BTA to know the responders (of 114 patients including only 57 were responders), and in the second phase, a clinical trial was conducted with the group of responders. We believe that it is a good thing to confirm that patients had a positive test before the clinical trial; although we thought it would have been more cost-effective if this diagnostic test had been performed with a local anesthetic to know the patients who were responders, thus lowering the costs of using BTA (this is what we usually do in daily practice). Another important point in this study is the inclusion criteria: They included patients with moderate to severe pain and a minimum pain duration of 8 months.
Regarding negative studies, we could say that it is very difficult to integrate clinical trial data from studies that were not favorable to BTA, because they all have a different design, starting with inclusion and exclusion criteria up to infiltration technique. In most of the clinical trials analyzed, we could find a decrease in VAS in both the BTA and the control groups without finding a statistically significant difference. It is likely that for this reason, authors such as Lew et al. suggest that the results obtained could be due to a placebo effect. Other authors do not agree with this conclusion and believe that the results obtained are due to the effect of needles, similar to that obtained in acupuncture (36).
There are other parameters that may be crucial in obtaining positive or negative results in clinical trials, such as patient follow-up time. Although most studies have a duration of 12 weeks, some studies have a short duration: Ojala et al. (4 weeks) and Qerama et al. (4 weeks), and Kwanchuay et al. (6 weeks). In all of them, the BTA was not superior to the NSS in controlling the MPS. A more evident example of the importance of follow-up time is found in the Benecke et al. study. They found no statistically significant differences at 4 weeks of follow-up in terms of neck pain improvement but found differences at 8 weeks.
We have found much variability in the site and the number of injections performed regarding the infiltration technique. Ferrante et al. infiltrated a maximum of 5 TP, Ojala et al. infiltrated up to 7 TP, while other authors such as Benecke et al. infiltrated 10 TP. On the contrary, Nicol et al. did not consider that TPs should guide infiltration but that infiltration should be performed in half the painful muscle thickness, not on TP. This variability of criteria again creates difficulty in interpreting the results.
The puncture of TPs was performed based on anatomical landmarks in most studies; few studies guided it by electromyography or fluoroscopy. In the present review, we found no clinical trial using ultrasound to guide TP infiltration. For future clinical trials, we consider that the use of ultrasound to optimize the effectiveness of infiltration may be interesting because it is a device we have in our usual clinical practice.
Although a standard dose to infiltrate the musculature is not yet agreed, we could affirm that doses less than 50 U as used in the study by Ojala et al. are ineffective. In contrast, doses higher than 200 U may have side effects, although clinical trials using maximum doses of 300 U and 400 U did not find any significant side effects and, if present, were related to muscle weakness in the infiltrated area or to flu-like symptoms, which were transient. In our opinion, it may be more important to assess the type of muscle to be infiltrated than to establish a standard dose of BTA per TP because bulky muscles such as iliopsoas or quadratus lumborum should have doses of 100 U and muscles such as trapezius 50 U. In the case of the study De Andrés et al., we consider that the use of higher doses of BTA would have been interesting because they used 50 U to infiltrate robust muscles such as the quadratus lumborum and the iliopsoas.
Dilution is another source of controversy; the first clinical trials used dilutions of 100 U/ml, while the last clinical trials used dilutions of 100 U/5 ml. We think it might be interesting to study whether the results are affected by using a concentrated higher or lower dilution, and according to the results, we could standardize dilutions to have more homogeneous clinical trials.
Based on the results of the Nicol et al. study where the placebo group had some residual effect of BTA given within 14 weeks prior to the beginning of the clinical trial; it may be interesting to perform studies determining when the second dose of BTA would be optimal to assess the possible increase in therapeutic effects.
We know that pain is difficult to assess because it has a biopsychosocial component; to try to be a little more objective in its assessment, some researchers use the algometer with which they have obtained very good results; we should bear in mind the use of scales and surveys that value the quality of life of patients that are no less important than VAS. Most studies inject BTA if the patient has a minimum VAS of 4, but some studies could be biased, such as the study of Ferrante et al., because they considered a very low VAS as inclusion criteria. In our opinion, it could be patients with mild pain who had a clinical improvement with the prescribed drug regimen together with physiotherapy, thus improving with conservative management even without having to administer BTA. Thus, the findings of this study may not have been favorable for BTA.
Finally, we would like to end with the statements common for all the studies analyzed. The first statement concerns the high cost of botulinum toxin; most authors believe that BTA should be reserved for cases in which conventional medical and interventional treatment fails (23,31).
CONCLUSIONS
Given the heterogeneous selection of patients, the large variability of BTA doses, the different techniques of infiltration of TP, the duration of the studies, and the lack of cost-effective analysis; We consider that more specific clinical trials with more homogeneous samples are needed to allow us to draw conclusions regarding treatment with BTA in the MPS.
REFERENCES