DOI: 10.20986/resed.2019. 3737-2019
SPECIAL ARTICLE
Key factors influencing bioavailability of opioides in the spinal cord for the management of acute pain
Factores clave que afectan a la biodisponibilidad de los opioides sobre la médula espinal en el manejo del dolor agudo
B. Mugabure Bujedo
Departamento de Anestesiología, Cuidados Críticos y Medicina del Dolor. Unidad del dolor, Manejo del dolor agudo y crónico. Hospital Universitario de Donostia, San Sebastián, España
Received: 8-04-2019
Accepted: 31-05-2019
Correspondence: Borja Mugabure Bujedo
mugabure@yahoo.es
ABSTRACT
Although there is no either ideal analgesic or route of neuraxial administration, clinicians alike continue to search for compounds with qualities which may approach this idea. However, it´s a demonstrated fact that spinal administration of an opioid drug does not always guarantee segmental and selective analgesia into the spinal cord. This point is valid due to partial reuptake of the drug to systemic blood circulation reaching specific brain opioid receptors, rather than the differences are explained by variations in the clearance rate from the cerebrospinal fluid. Published evidence from either human or animal experimental studies indicates that bioavailability in the spinal cord biophase is negatively correlated with liposolubility. Therefore, opioid spinal cord bioavailability is higher for hydrophilic opioids like morphine, than for lipophilic ones such as fentanyl, sufentanil or alfentanil.
Keywords: analgesics, opioids, biological availability, opioid receptors, acute pain, spinal cord.
RESUMEN
Aunque no existe un analgésico ni una vía de administración neuroaxial ideal, los clínicos continúan buscando compuestos con cualidades que puedan acercarse a esta idea. Sin embargo, se ha demostrado que la administración espinal de un medicamento opioide no siempre garantiza una analgesia segmentaria y selectiva en la médula espinal. Este punto es cierto debido a la recaptación parcial del fármaco a la circulación sistémica sanguínea que alcanza receptores opioides cerebrales específicos, además de las diferencias que se explican por las variaciones en la tasa de eliminación del líquido cefalorraquídeo. La evidencia publicada de estudios experimentales en humanos o animales indica que la biodisponibilidad en la biofase medular está correlacionada negativamente con la liposolubilidad. Por lo tanto, la analgesia espinal es mayor para los opioides hidrófilos como la morfina, que para los lipófilos como el fentanilo, el sufentanilo o el alfentanilo.
Palabras clave: analgésicos, opioides, biodisponibilidad medular, receptores opioides, dolor agudo, médula espinal.
INTRODUCTION
Opioid drugs have been used to achieve spinal analgesia for more than a century. Currently, epidural or intrathecal injections have a key role in controlling postoperative pain. Since the discovery of the endogenous opioid system three decades ago, the use of opioids has become widespread for obstetric analgesia and for the treatment of postoperative pain and chronic cancer pain (1). Presumably, the presence of any opioid in the human body will produce a certain level of analgesia, but it is not necessarily true that the neuraxial administration guarantees its action at the level of the spinal cord and, even if so, that the effect is better than analgesia achieved through other systemic routes of administration such as intravenous or subcutaneous. In fact, not all opioids administered via neuraxial produce spinal analgesia. Therefore, to use opioids effectively for this type of analgesia, it is important to understand the physiology and clinical pharmacology of these active ingredients, focusing on those producing analgesia by an intrinsic spinal mechanism. In summary, we could state that tests in animal and human trials indicate that hydrophilic opioids, such as hydromorphone, diamorphine and morphine, bind more strongly to specific receptors within the dorsal horn of spinal cord, which is understood as selectivity and bioavailability at the spinal cord, than lipophilic opioids, such as alfentanil, fentanyl and sufentanil. This fact is attributable to the differences in the pharmacokinetic and pharmacodynamic properties of both groups. It is more difficult for lipophilic opioids to reach and remain at sufficiently high concentrations at the site of action or spinal biophase in Rexed’s lamina II, due to its sequestration in epidural fat and rapid plasma clearance from epidural and intrathecal spaces, which results in analgesia with a limited extension and duration, as well as the appearance of early supraspinal side effects. In contrast, morphine and other hydrophilic opioids have very different properties, which include greater bioavailability and this means that, administered neuraxially, their residence time in their effect compartment is greater and, therefore, it is a suitable option for treatment of acute pain (2).
The purpose of this narrative review was to determine the key factors to explain which opioids reach sufficiently high concentrations to produce selective analgesia when administered epidurally or intrathecally and also to make some recommendations on their rational use for the treatment of postoperative pain. With this purpose, a search was conducted in Ovid / Medline / Embase / Cochrane Database to identify all articles published until December 2018 using the keywords: analgesics, opioids; bioavailability; opioid receptors; acute pain; spinal cord.
The search terms included the following:
Acute postoperative pain ± morphine, fentanyl, alfentanil, sufentanil
Neuraxial block ± opioid receptors. Opioids drugs have been approached in systematic reviews, meta-analyzes or Cochrane reviews. For agents who have not undergone such a systematic combination of data, they were individually assessed to conduct a narrative review.
For spinal bioavailability, relevant studies were obtained through a Medline search and they were individually reviewed to identify any study that might have been lost in the main search. Additional material was retrieved by manually reviewing references of relevant articles identified with a higher impact level and previous references.
All references finally included were chosen by the author in order to focus the review on the intended terms.
MECHANISMS CONTROLLING THE SPINAL DISTRIBUTION OF OPIOIDS
All opioid drugs produce analgesia by using the same molecular mechanism, that is, through a decrease in the excitability of nerve cells. To achieve this, opioids need to bind to the G protein, inhibit the adenylate cyclase enzyme and stimulate the activation of potassium channels, as well as achieve inhibition of voltage-dependent calcium channels. Given this common analgesic mechanism, it is logical to wonder why there are so many clinical differences between opioids. The explanation lies in its pharmacokinetic and pharmacodynamic characteristics and what they imply for the selection of the optimal postoperative drug regimen (3). Consequently, during the last three decades, the scientific effort in this field has focused on conducting controlled clinical trials to determine which of the available opioids is most suitable for spinal use. It is often assumed that, at least in some aspects, the neuraxial administration of opioids would produce better analgesia compared to the use of other parenteral routes and also less adverse effects, including respiratory depression. Unfortunately, this is not always true, since many opioids can reach the upper brain centers through the cerebrospinal fluid (CSF) or by their reabsorption into the bloodstream, which results in a very low spinal bioavailability (4). (Table I).

Experimental studies in animals
Epidural diffusion
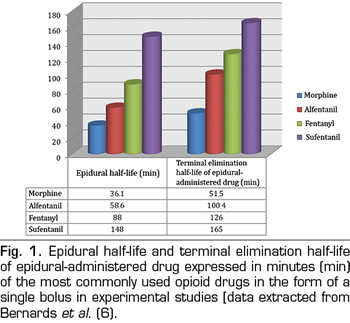
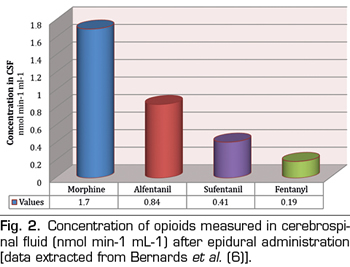
For opioid drugs to have spinal analgesic effect they must move from the epidural space to the specific site of action in the gray matter of the dorsal horn of the spinal cord. Therefore, one of the most important factors to consider is the ability of drugs to redistribute to neighboring tissues, spreading away from the epidural space and specifically crossing a range of barriers, such as meninges, CSF and spinal white matter. In general, all substances diffuse following a concentration gradient; therefore, any opioid placed in the epidural space will tend to spread to surrounding tissues. However, the speed and distance at which a drug moves in a specific tissue depends on the volume of that tissue and its physical and chemical features in relation to those of that drug. In particular, the laws of thermodynamics favor the accumulation of hydrophobic drugs in tissues with similar properties. Given this, fentanyl and sufentanil can be expected to diffuse preferentially in epidural fat instead of in CSF, so they will no longer be available to opioid receptors in the spine. Epidural fat, located mainly in the lateral and posterior sides of the epidural space, dampens the pulsation of the dural sac and facilitates the movement of the periosteum of the spinal canal, during flexion and extension of the spine. Given its lipophilic nature, epidural fat behaves as a reservoir of lipid soluble drugs, which can produce a sustained release of the drug and prolonged analgesia (5). In an animal model (pigs), Bernards et al. (6) explored the administration of different epidural opioids by boluses (morphine, fentanyl, alfentanil and sufentanil) and measured their concentration over time in the epidural space and fat, intradural space, central venous plasma and epidural venous plexus. They demonstrated that the residence time in the epidural space and the concentration in the epidural fat were directly correlated with the liposolubility of the drug, both being higher for sufentanil and fentanyl and lower for morphine (Figure 1). Specifically, it was found that the cumulative concentration in epidural fat was 32- and 20-fold higher for fentanyl and alfentanil respectively than for morphine and, consequently, smaller amounts of those opioids reach the spinal biophase. In addition, as might be expected, the proportion of drug that reached the CSF was higher for morphine than for lipophilic opiates, which were sequestered within the fat (Figure 2). Finally, they found that alfentanil reached the highest plasma concentration in the opioid group due to its rapid elimination into the central blood compartment.
Meningeal diffusion
Experimental studies suggest that the primary mechanism by which opioids reach the CSF is simple diffusion through the meninges, aided by the kinetic energy of the pulsatile flow of the CSF associated with the secondary movement of the spinal cord. Correctly, it has been observed that diffusion through arachnoid villi in the roots of the spinal cord (7) and the radicular arteries involved in its vascularization (8) do not participate in this process. Although there are differences between opioid drugs, they do not appear to be important in redistribution from the epidural to the deepest subarachnoid spaces.
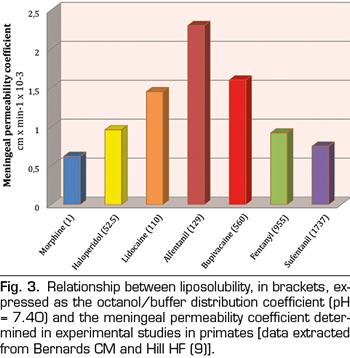
There is a biphasic relationship between drug solubility and arachnoid permeability (9). At first, the permeability increases with increasing lipid solubility, but only up to moderate values (approximately 125) of the octanol/buffer distribution coefficient. At higher values, permeability decreases significantly with solubility. In line with this, the coefficients of the meningeal permeability of morphine (M) and sufentanil (S) are similar, 0.6 and 0.75 respectively, but their octanol/buffer distribution coefficients are very different, 1 (M) and 1787 (S) (Figure 3). The explanation to this biphasic relationship lies in the fact that drugs must first cross the lipid bilayers of arachnoid cells and then through the fluids of extracellular and intracellular spaces. Highly lipophilic drugs complete the first step easily but the second one with difficulty, while for the hydrophilic drugs the opposite mechanism is more correct. This, together with the fact that arachnoid is the main barrier to meningeal permeability (90%), explains why drugs with intermediate lipid solubility values (lidocaine or alfentanil) achieve better transfer rates globally in this type of tissues.
Therefore, and although the meninges do not play an essential role as a selective physical barrier for spinal opioid diffusion, it is, however, worthy of highlighting its essential function as a place for the regulation of intrathecal drug absorption, given the dense network of capillaries on the inner surface of the dura mater. This conclusion is based on the results of two experimental studies in animals: Kozody et al. (10) showed that spinal administration of adrenaline and phenylephrine significantly reduced blood flow to the dura mater without affecting the flow of the spinal cord and Bernards et al. (11) found that the administration of adrenaline together with a hydrophilic epidural opioid, such as morphine, reduced its plasma clearance and concluded that this was probably due to the constriction of blood flow at the dura mater.
Intrathecal transport
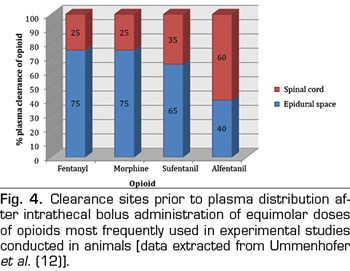
Without differences between the baricity, the volume of drug administered, the injection site or the kinetic energy provided by the injection itself, the opioids that reach the CSF should behave similarly. Ummenhofer et al. (12) found that the volume of distribution of opioids injected intrathecally was directly related to their lipid solubility, the volume being 40-fold greater for sufentanil than for morphine. This means a rapid redistribution of the intrathecal compartment to more lipophilic environments, and in that study, the most crucial route of clearance was defined through the meninges into the epidural space, except for alfentanil (Figure 4). The implication regarding lipophilic opioids is that their rostral propagation through the CSF is therefore limited and their spinal bioavailability is relatively weak.
The primary mechanism for drugs to spread through the CSF is the movement of the fluid itself. The associated energy comes from the pulsatile flow in the central nervous system. This transiently increases the volume of the brain and to a lesser extent that of the spinal cord, forcing the CSF in the caudal direction on the dorsal surface and cranial direction through the ventral one. This effect is greater in areas near the cervical spinal metameres C4-C5, and minimal in the lower lumbar area. As the CSF moves, it carries any molecule suspended in it, and this mechanism does not induce any difference between opioids, which can also spread by diffusion. The simple diffusion rate of any molecule in an ideal fluid is proportional to the temperature of the liquid and inversely proportional to the square root of its molecular weight. However, since the temperature of the CSF is constant and the square root of the molecular weight of different opioids is similar and ranges between 17 and 20, the theoretical diffusion rates are identical for all opioid drugs and cannot explain the differences observed in its spread through the CSF. Differences are explained by the variations in the CSF clearance rate, since if a drug disappears quickly from this compartment, there is, by definition, little rest to spread rostrally and, in turn, produce spinal analgesia. For example, in humans, the sufentanil clearance rate (27 μg/kg/min) is almost 10-fold faster than that of morphine (2.8 μg/kg/min). Therefore, morphine remains in the CSF for longer and it is more likely to spread to the brain and cause other supraspinal effects, such as sedation and respiratory depression (2-4).
In a fascinating experimental study conducted in animals (pigs) (13), it was discovered that after continuous intrathecal infusion of bupivacaine and baclofen there was a very poor internal redistribution through the CSF and the differences in drug concentrations were very important between the dorsal and anterior surfaces of the spinal cord, due to a rostral-caudal gradient. This last gradient, previously observed for albumin and glucose, is attributable to a small transfer of kinetic energy from the systole phase of the cardiac cycle and to the high degree of internal anatomical compartmentalization of this intradural space. After 8 hours of infusion, the drugs had extended no more than 7 cm from the injection site and drugs were detected at this distance at much higher concentrations in the dorsal than in the anterior surface of the spinal cord. Therefore, the factors that are clinically important in determining spinal analgesia are the clearance rate of CSF drugs and the amount of drug available in the spinal biophase, as well as the mean elimination half-life. The bioavailability of medications will be greater when it adheres directly to the posterior horn of the spinal cord instead of being distributed through the blood or epidural space. We must also determine which fraction of the analgesic effect can be attributed to a spinal action and which fraction to a supra spinal action, as well as if the latter is necessary for the final analgesic observed effect (2-6).
Spinal analgesic action
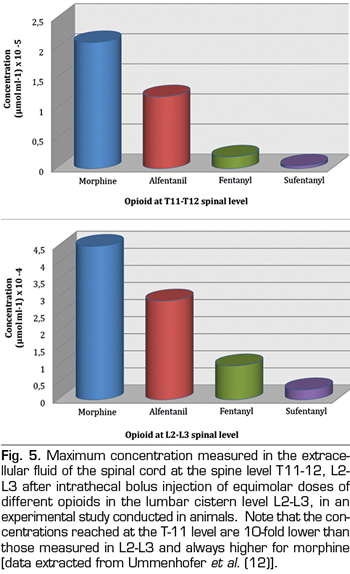
Finally, the last step for opioids already present in the spinal cord is to cross white matter and join specific receptors in the gray matter. In a classic study conducted at the 70s, von Cube et al. (14) injected radioactively labeled morphine, hydromorphine and fentanyl into the CSF of the lateral ventricles of rabbits and measured the distance reached in neighboring tissues of the central nervous system over time. They found that the three drugs penetrated 700 µm in the first 7 minutes, but, as time went by, fentanyl did not advance further and it was removed from brain tissue at 120 minutes. In contrast, the distribution of morphine and hydromorphine continued and at the end of the study, after 5 hours, morphine had reached a tissue depth of about 3000 µm. An even more surprising observation was that fentanyl had a greater affinity for white matter, compared to water soluble drugs, which had more affinity for gray matter. More recently, in the late 1990s, this fact was confirmed in an experimental model in pigs (12), with intrathecal administration of morphine, alfentanil, sufentanil and fentanyl, in equimolar doses, and subsequent measurements of drug concentrations in the extracellular space of the spinal cord. The exposure to morphine was greater than that of all lipophilic drugs, since it had a concentration 3-fold higher, both in the lumbar spine at the injection level (L2-L3) and also in the thoracic spine (T11-T12) (Figure 5). The explanation of these observations is that white matter is mainly composed of axonal plasma membranes surrounded by layers of Schwann cells and, consequently, white mater has a lipid content of about 80% and, therefore, a higher affinity for lipophilic opiates. Since gray matter does not contain myelin, it is relatively hydrophilic and therefore has a higher affinity for morphine. Bernards CM (15) reviewed in 2004 experimental studies on animals that measured opioid concentrations in the epidural and intrathecal spaces, the spinal cord and surrounding tissues after epidural or intradural administration. His study concluded that these data in animals help us to understand the findings of several clinical trials on the analgesic effect of lipophilic opioids, that is, that the result is partly, or even exclusively in some cases, due to plasma uptake and redistribution to central opioid receptors of the brain and not due to their selective spinal action.
Recently, the potency and some adverse effects of series of small opioid peptides called DALDA peptides (DMT-DALDA, dDALc, dDALcn, dDAL-TICP and dDAL-TIPP) after intrathecal bolus administration have been studied in rats. Special emphasis was placed on separating adverse effects from antinociceptive effects. These peptides are hydrophilic, such as morphine, which may make it possible for them to be used to produce a long-lasting effect after a single intrathecal bolus dose. Because continued intrathecal administration is somewhat problematic in clinical practice, especially after adverse effects related to the use of spinal catheters, prolonged analgesia after a single dose would be a highly desirable property within spinal analgesia. Obviously, before any clinical application, more research is needed on its efficacy and safety. In this study, experimental animals were treated with intrathecal morphine for five days before the administration of DMT-DALDA, and it was observed that the analgesic effect was not reduced, occurs with morphine, as a result of tolerance development. This asymmetric tolerance is interesting, as it could indicate that the peptide has a better intrinsic effect compared to morphine and opens a promising line of investigation (16).
Experimental spinal diffusion studies in humans
Classic Experimental Studies
Opium is the dried latex obtained from Papaver somniferum and the use of the plant itself has a long history in the development of humanity. The widespread medical use of unprocessed opium was a common practice over the centuries until morphine was first discovered in 1804 by a German pharmacist, Friedrich Sertürner, who first distributed this medication in 1817 (17). The Romanian surgeon Racoviceanu-Pitesti, reported on his experience using a mixture of intrathecal cocaine and morphine in 1901, and made the first publication on the use of opioids in spinal anesthesia (18). After the development of new opioid agonists/antagonists in the 1940s, scientists began to believe that there should be other natural binding sites in the brain for these opioid-like drugs. These problems were overcome in 1973 when Pert and Snyder characterized even better the properties of this specific binding for opiates in nerve tissue (19). These receptors were not only found in the brain, but they were also found in the gelatinous substance of the spinal cord. Later, Fields et al. found that the primary afferent tissue of the dorsal root and the dorsal horn of the spinal cord contained multiple types of opioid receptors (20). The year 1975 was crucial in relation to the discovery of endogenous opioids (encephalin) by Kosterlitz et al. (21). In addition, Yaksh et al. (22) showed that the direct application of morphine in the spine of rats by means of a chronic intrathecal catheter produced analgesia, and this practice became a reality when Wang et al. successfully used intrathecal injection of morphine bolus in humans (23). They randomly assigned eight patients with pain due to genitourinary cancer disease to receive either saline alone or together with 0.5-1.0 mg of morphine. Six of them reported complete pain relief after morphine injection, which lasted for 12-24 hours. The publication of Behar et al. in The Lancet in 1979 was the first article published on the use of epidural morphine at doses of 2 mg for both acute and chronic pain treatment (24). These authors reported that they managed to relieve pain for 6 to 24 hours in 10 patients and they suggested that there was a direct spinal effect on specific spinal cord receptors. Therefore, it required more than a century until the use of neuraxial opioids became a clinical routine to achieve analgesia in acute and chronic patients.
After administration within the vertebral canal, opioids are complexy distributed, which can be described by a multicompartmental model. A drug directly deposited in, or reaching, the intrathecal space simultaneously undergoes a movement in a caudal direction on the dorsal side of the spinal cord, and then goes up the ventral side toward the head. Subsequently, during its diffusion in the spinal cord, it binds to specific receptors in the gray matter and also to non-specific receptors in the white matter. It also diffuses in the opposite direction, towards the epidural space, being sequestered in the epidural fat and, finally, the diffusion into the blood of each of these compartments. The balance between all these processes, described in relation to the spinal bioavailability of the drug, determines its clinical characteristics and its general analgesic efficacy (25). Some evidence shows that respiratory depression, sedation and pruritus are associated with the degree of rostral migration of opioids in the CSF and the timing of these side effects varies between lipophilic and hydrophilic opioids. It has been estimated that the morphine administered in the lumbar cistern reaches the cisterna magna in 1-2 hours and the fourth and lateral ventricles in 3-6 hours. On the other hand, lipophilic opioids can also have a central effect, since they are distributed more quickly through the bloodstream, thus reaching the central nervous system. In addition, although to a lesser extent, distribution also occurs through the CSF, with traces of opioids, including sufentanil, found in the magna cistern only 30 minutes after lumbar intrathecal administration (26). It has been found that fentanyl reaches a maximum cervical CSF concentration as early as 10 minutes after lumbar epidural administration, at an average of 10% of maximum lumbar CSF concentrations, with high individual variability (27). In this study, fentanyl was found to penetrate very poor into the cervical and lumbar CSF after intravenous administration, and only 4 of the 60 CSF samples studied had detectable concentrations of fentanyl. In contrast, after lumbar epidural administration, the penetration of fentanyl through the dura mater was of greater magnitude and rapidity. To investigate the cephalic spread of opioids, intrathecal injections of 50 μg of fentanyl (F) along with the same dose of morphine (M) were administered to a group of healthy volunteers, in the lowest palpable interspace (L5-S1), and CSF samples were collected at the highest possible level in the lumbar space (L2-L3) that were analyzed up to 120 minutes after injection. It was found that both drugs reached their maximum concentration at the most cephalic point at similar time points (41 ± 13 min for F and 57 ± 12 min for M), while the ratio M:F concentration increased from 2:1 after 36 minutes to 4:1 after 103 minutes; in addition, no speed constant was correlated with weight, height or volume of the CSF. These findings were explained using a simple pharmacokinetic model with relatively high individual variability. The authors concluded that fentanyl is eliminated more quickly than CSF morphine because it has a greater clearance, although the distribution in the first hour after administration is similar for both drugs (28).
While the administration of spinal opioids may clearly be an effective analgesic technique, there is a widespread misconception that any epidural or intrathecally administered opioid will always produce analgesia by a selective spinal mechanism. This is not entirely true, since several opioids that are commonly administered by the spinal route can also produce early analgesia by absorption in the systemic circulation, with later redistribution to opioid receptors of the brainstem. This is more applicable for lipophilic opioids because their minimum effective analgesic concentration (MEAC) is too low and can be easily achieved in plasma after neuraxial administration (0.03 μg/mL for sufentanil and 0.63 μg/mL for fentanyl). Therefore, in some cases, the analgesia produced may not be superior to that produced by intravenous administration. On the other hand, hydrophilic opioids, such as morphine (MEAC 9-30 μg/mL), remain for a sufficient time in the CSF, so that cephalic recirculation causes a delay in either analgesia or adverse effects (2-6). That is why, there are still controversies regarding the selective spinal action of lipophilic opioids, such as fentanyl, via epidural route. In an experimental study of pain conducted in volunteers by Ginosar et al. (29), epidural fentanyl caused segmental analgesia when administered as a bolus and not segmental or systemic when administered as a continuous infusion. The response to the maximum tolerable pain was evaluated over a period of 420 min with electrical and thermal stimuli in two regions, in the head and leg (supraspinal and spinal, respectively). Plasma concentrations of fentanyl were measured and were only found when MEAC could be reached after a continuous infusion at high doses. The findings were mostly consistent with the previous studies reviewed and they were explained by the higher level of fentanyl that reached the biophase spinal cord in the bolus group. It has been suggested that this effect is due to the concentration gradient reached between the epidural zone and the intrathecal space after bolus administration and not reached under continuous infusion. The dose that fentanyl would need to reach to produce spinal anesthesia has been estimated at approximately 10 μg/mL. Consequently, if this opioid is combined with an infusion of LA (Local Anesthetic) at a dose of 2-5 μg/mL in the routine postoperative clinical practice, we expected to achieve an improvement in analgesia by decreasing the dose of AL, with a potential systemic effect and, therefore, an additive effect instead of a spinal synergistic one (30). Therefore, these findings may help resolve the controversy surrounding the site of action of epidural fentanyl according to its mode of administration, but further trials are needed to evaluate this claim.
Regarding this issue, Mather and Cousins (31) suggested that it is reasonable not to think about supraspinal and spinal mechanisms in terms of a dichotomy. Despite comments on lipophilic drugs, such as fentanyl distributed in epidural fat, numerous studies with epidurally injected opioids and LA (remember that fentanyl and bupivacaine have similar physicochemical properties) have shown that systemic absorption has a profile of blood concentration similar to that of intramuscular injection. The biphasic absorption patterns found can be interpreted as a “portion” of the dose that is absorbed reasonably quickly with a half-life of about 5 to 10 minutes, generating the “maximum” arterial blood concentration in about 10-20 minutes after the injection. The remaining “portion”, presumably distributed in fatty tissues, is absorbed more slowly with a half-life of several hours, which maintains drug concentrations in the blood compared to intravenous administration. Therefore, the drug transported by blood will bind both supraspinal and spinal receptor sites in proportion to the distribution of cardiac output, in addition to the drug administered by local diffusion mechanisms. Although the amount of drug in plasma that would bind supraspinal opioid receptors may be small after epidural administration compared to intravenous injection, it should be remembered, as noted above in the pharmacology of spinal opioids, that dual opioid actions (spinal and supraspinal) have a reinforcing action that is relevant to both the agonist and the clinical antagonism.
Computational and in vitro studies
In experimental studies in humans, it was shown that both the heart rate and the pulse volume in the CSF of the patient decisively influence the distribution of the drug after intrathecal administration. The throbbing effect on the CSF due to the frequency and cardiac systolic volume was investigated for a bolus injection of a model drug at L2 vertebral level. Distribution profiles of the drug throughout the entire spine were calculated for different heart rates: 43, 60 and 120 beats per minute (bpm), and various injection volumes: 1, 2 and 3 mL. At excited heart rate (120 BPM), drugs disperse faster than a resting individual (60 BPM). A simulated case of brachycardia (40 BPM) results in the smallest volume of distribution. Doubling the heart rate (from 60 to 120 bpm) caused a 26.4 % decrease in peak concentration in CSF after injection. Doubling the CSF stroke volume diminished the peak concentration after injection by 38.1 %. Calculations show that potentially toxic levels of drugs due to injection mode can be avoided by changing the infusion rate. The use of slower infusion rates could avoid high maximum concentrations in the CSF while maintaining drug levels above the therapeutic threshold (32). In another recent study, the authors acquired anatomical magnetic resonance imaging (NMR) data and velocity measurements in the CSF with KINETIC NMR for two voluntary subjects. Using reconstructions from NMR data, they also introduced a subject-specific computer model to predict the spread of the drug. The results found were surprising. The velocity measurements in three spinal regions of interest were reasonably consistent with the simulated flow fields in a subject-specific computer mesh. The infusion of drugs in the form of a simulated multibolus theoretically located the drug in the upper cervical and thoracic region. Furthermore, the injection of drug in the form of continuous infusion and the injection of a single bolus were advantageous for concentrating the drug at the determined point of injection into the lumbar spine. The authors presented potential guidelines that take into account the specific kinetics of the drug for the final uptake at a tissue point, which influences the rate of dispersion of the drug in the model (33). This study also quantifies how injection kinetics radically change the specific location of the final action of the drug. Due to the different tissue uptakes of 3 agents of usual use (morphine, sufentanil, alfentanil), a higher fraction of sufentanil and morphine remained in the CSF, along the neuroaxis, which induced a stronger action in the upper cervical region. In the chronic clinical treatment of cervical pain, these agents could be used with more power, while preliminary simulation shows that alfentanil could be more suitable for low back pain, according to our high uptake rate by the tissues surrounding the injection site. However, morphine would be more easily distributed along the entire spinal axis for a pain associated with cancer.
CONCLUSIONS
Professor Bernards CM used to say: “Each opioid injected into the human body from the right ear to the left foot will induce an analgesic effect due to the systemic distribution to brain receptors. Therefore, the spinal administration of an opioid does not always guarantee a selective spinal effect”. There is a consensus in the scientific community that opioids are the most potent central-acting drugs available in the arsenal to treat both postoperative and chronic pain. However, the debate continues on whether the neuraxial pathway maintains an efficacy/safety profile to obtain selective spinal analgesia (1-6). Experiments in animals or humans support the theory that bioavailability in the spinal biophase is inversely proportional to the solubility of the drug, which is higher in hydrophilic opioids than in lipophilic opioids (7-15). Therefore, epidural morphine is considered very useful in patients with acute and postoperative pain but its short duration of action for less than 24 hours limits the usefulness of bolus injections, and adverse effects are associated with increasing the dose. For these reasons, the administration of morphine by continuous infusion in combination with local anesthetics has been recommended (25-31). Morphine can be considered the opioid with the best set of characteristics for spinal administration, but this does not mean that it is the ideal drug in all situations. In particular, morphine should not be used in outpatient surgery or in patients with high cardiorespiratory risk, and causes delayed supraspinal side effects, such as respiratory depression, which means that morphine cannot be recommended for general use and patients should be carefully selected (34). All intrathecal opioids cause some of their analgesic effects through spinal selectivity, but lipophilic drugs can also reach higher brain centers through absorption in the blood and, therefore, they can cause both early sedation and respiratory depression, within half an hour after beginning its administration until 2-4 hours of elimination half-life (35).
Finally, we must remember that all patients receiving neuraxial opioids should receive adequate surveillance and monitoring focused on ensuring adequate ventilation, and respiratory rate, as well as an appropriate level of awareness, for a period equivalent to the half-life of the drugs. Therefore, this period is about 4-6 h for lipophilic opioids and 12-48 h for morphine in the case of bolus injections, and the full duration of treatment when continuous infusion is necessary, for achieve safe analgesic control in patients with postoperative pain (36).
REFERENCES