DOI: 10.20986/resed.2020.3717/2018
REVISION
Peripheral acting mu opioid receptor antagonists in the treatment of opioid-induced constipation: review
Antagonistas periféricos de los receptores opioides mu en el tratamiento del estreñimiento inducido por opioides: revisión
A. Libran Oriol1, C. Cruz-Sequeiros2, A. Luque-Blanco2 and J. Porta-Sales3
1Servicio Cuidados Paliativos. Consorci Sanitari de Terrassa. Barcelona, España. 2Servicio de Soporte y Cuidados Paliativos. Institut Català d’Oncologia-Girona. España. 3Servicio de Cuidados Paliativos. Institut Català d’Oncologia-L’Hospitalet de Llobregat. Instituto de Investigación Biomédica de Bellvitge (IDIBELL). Facultad de Medicina y Ciencia de la Salud. Universitat Internacional de Catalunya. Barcelona, España
Received: 11-12-2018
Accepted: 13-01-2019
Correspondence: Josep Porta-Sales
jporta@iconcologia.net
ABSTRACT
The opioid induced constipation (OIC) is an emerging clinical problems that worsen the patients’ quality of life requiring opioids for their pain relief. Many drugs have been launched as Peripheral Acting Mu Opioid Receptor Antagonists o PAMORAs (metylnaltrexone, alvimopam and more recently naloxegol), which antagonize the peripheral effects of opioids without affecting the opioids analgesia. Metylnaltrenone and naloxegol have been licensed for the treatment of CIO, meanwhile alvimopam is approved for the recovery of postoperative ileus after major abdominal surgery. All PAMORAs have shown clinical efficacy but is a matter of debate wich should be their role in the manangement of the CIO. The available information about PAMORAs is reviewed and informed strategy on the use of these drugs is proposed.
Key words: Pain, PAMORA, opioid induced constipation (OIC), metylnaltrexone, alvimopan, naloxegol.
RESUMEN
El estreñimiento inducido por opioides (EIO) constituye un problema clínico emergente que empeora la calidad de vida de los pacientes que requieren el uso de opioides para el manejo del dolor. Diferentes fármacos se han comercializado como antagonistas de los receptores opioides Mu periféricos, conocidos con el nombre de Peripheral Acting Mu Opioid Receptor Antagonists o PAMORA (metilnaltrexona, alvimopam y más recientemente naloxegol), que permiten la antagonización de los efectos periféricos de los opioides sin interferir en su efecto analgésico. Tanto metilnaltrexona como naloxegol han sido aprobados para el tratamiento del EIO, mientras que alvimopan está aprobado para la recuperación gastrointestinal después de resección intestinal con anastomosis primaria. Todos ellos han mostrado su eficacia clínica, pero es debatido el papel que han de tener en la estrategia global del manejo del EIO. Se revisa la información disponible sobre los PAMORA y se propone estrategia de uso clínico.
Palabras clave: Dolor, PAMORA, estreñimiento inducido por opioides (EIO), metilnaltrexona, alvimopan, naloxegol.
INTRODUCTION
Constipation is a frequent symptom, described in different chronic diseases (such as cancer, heart failure, chronic obstructive pulmonary disease, renal failure or degenerative neurological diseases), estimating its prevalence around 50% (1,2), of which 20% of patients will present with severe constipation at some point during the evolution of the disease (3). In fact, the description of its prevalence is greatly affected by the factors considered in its evaluation and definition (4). Even in the absence of a globally accepted definition of constipation, the criteria proposed by the Rome Foundation have been gaining acceptance; thus, the ROME III criteria of constipation combine objective criteria (such as stool frequency and stool consistency) together with subjective criteria (such as straining, the feeling of incomplete bowel evacuation and the feeling of anorectal obstruction) (5).
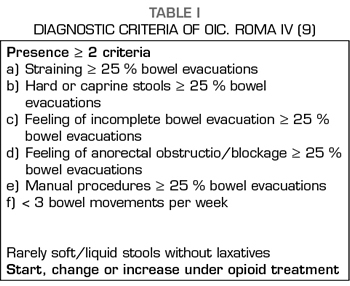
Constipation is a multicausal symptom, in which pharmacological, metabolic or other causes contribute to its appearance; this has been especially evident in cancer patients (6). The role of opioid treatment has been recognized as one of the most important factors in the etiology of constipation in cancer patients, considered related in 84.5% of cases and with a moderate to intense intensity in 63% of them (7). The recognition of the relevance of the role of opioids has led to the recognition of opioid-induced constipation (OIC) as a specific entity in the new ROMA IV criteria (8,9), whose diagnostic criteria are shown in Table I.

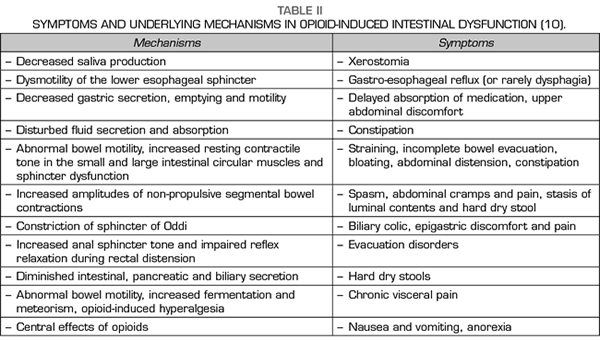
Constipation is perhaps the most obvious symptom of a whole set of changes produced by opioids on the digestive system, known as opioid-induced bowel dysfunction, characterized by slower GI passage, decreased secretion of intestinal fluids and increased sphincter tone (10-12) (Table II). The usual treatment of OIC is based on the use of laxatives, being the common and the most effective the combination of laxatives, often osmotic and stimulants being habitual and more effective (13,14), but their action is merely symptomatic and does not affect other symptoms and problems resulting from the action of opioids on the GI tract.
In recent years, drugs called Peripheral Acting Mu Opioid Receptor Antagonists), or PAMORA, have become available, which allow the antagonization of the peripheral effects of opioids without interfering with the analgesic effect of opioids. The present study tries to review the information available on the use of PAMORA and its role in the treatment of OIC.
PERIPHERAL ACTING MU OPIOD RECEPTOR ANTAGONISTS OR PAMORAS
Currently, three PAMORA are marketed: methylnaltrexone, alvimopan and naloxegol. The commercialized oxycodone-naloxone combination bases its mechanism of action and efficacy on the low bioavailability of naloxone orally (15), which allows peripheral antagonization of the Mu opioid receptors, without affecting the central analgesia if not exceeding 40 mg of naloxone per day. Administration of higher doses and reversal of analgesia has been reported (16); therefore, the oxycodone-naloxone combination should not be considered PAMORA, but rather an opioid receptor antagonist with limited systemic absorption (17).
Regarding the buprenorphine-naloxone combination used in the treatment of opioid addiction detoxification (18), studies regarding its benefit in pain relief in patients with pain and addiction (19,20), and a potential benefit in the OIC (21) are available.
Methylnaltrexone
Methylnatrexone bromide (MTNX) is a quaternary amine that is formed from N-methylation of naltrexone. The positive charge of the ammonium group increases its polarity and reduces its fat solubility, decreasing its passage through the blood-brain barrier, and acts as a peripheral antagonist of the Mu receptors, reducing the oral-cecal transit time without reversing analgesia (22).
Pharmacokinetics
Subcutaneous methylnaltrexone is rapidly absorbed, showing its maximum concentration (Cmax) after 30 minutes. Its subcutaneous bioavailability is 82%. After administration at both single and repeated doses of 0.3 mg/kg/IV, its elimination half-life is ≈2.5 hours (23). Only 10% is metabolized by glucuronidation in the liver and no interference with the cytochrome P450 system is found (24). About 50% is eliminated unchanged by the kidney and 40% is eliminated in stools (24). In patients with a creatinine clearance ≤ 30 ml/min/1.73 m2, a dose reduction of 50% is recommended. In terminal renal failure/dialysis or severe liver failure is not recommended. Age does not seem to have a significant effect on the pharmacokinetics of MTNX (25). Zacnya et al. (26), in a randomized double-blind, placebo-controlled crossover study conducted in 29 healthy volunteers, the authors found that MTNX at a dose of 0.45 mg/kg (approximately twice the usual therapeutic dose) administered subcutaneously presented myosis, suggesting some level of central action.
Clinical trials
Portenoy et al. (27), in a phase II study in which doses of MNTX of 1, 5, 12.5 and 20 mg subcutaneously every other day were compared, in patients with cancer or advanced AIDS, it was shown that laxation was achieved for doses ≥ 5 mg without being analgesia compromised. Thus, for a population of average weight of 64 kg, the MTNX dose would be between 0.08 and 0.20 mg/kg. Mori et al. (28), in a single-arm, uncontrolled phase II study, 12 patients with cancer and expected survival> 3 months were evaluated; 83.3% of the patients had a spontaneous bowel movement within the first 48 hours after the administration of MTNX, with further improvement of stool consistency. In another phase II, placebo-controlled study, in patients with acute OIC after orthopedic surgery under opioid treatment, it was found that 38.9% of patients in the MTNX arm had a spontaneous bowel movement within 4 h after administration, compared to the 6.7% of the placebo group (p = 0.046) (29).
Thomas et al. (30) conducted a phase III multicenter, randomized, double blind and placebo-controlled study. A total of 133 patients were randomized: MTNX 0.15 mg/kg every other day vs. placebo. The two main co-objectives were deposition within the first 4 hours after the first administration of MTNX and bowel movement within the first hours after the second or successive doses of MTNX. The MTNX group was more effective than placebo for both objectives. El 48 vs. 15 % (p < 0.0001) of the patients presented a bowel movement within the first 4 hours after the first administration of MTNX and placebo, respectively. For the following doses, 52% of the patients of the MTNX group had bowel movements within the first 4 h compared to the 8% of the placebo group (p <0.0001). In the MTNX group, stool consistency and straining also improved. Similar results were found in a randomized, double blind and placebo-controlled phase III study (31) that included 460 patients with non-cancer pain. Patients were randomized in three branches: placebo, MTNX 12 mg/sc/24 h and MTNX 12 mg/sc/48 h; the percentage of patients with bowel movements within 4 hours after the first dose were: 9.9%, 33.3% and 35.1%, (p <0.001), respectively. It also improved stool consistency and straining in the arms of MNTX. Another placebo controlled phase III study (32) evaluated 154 patients with advanced disease, a single subcutaneous dose of MTNX (0.15 mg/kg o 0.3 mg/kg) or placebo was administered; 62% and 58% of the patients with MTNX had a spontaneous bowel movement within the first 4 h, respectively, while in the placebo group only 14% of the patients showed this effect (p <0.0001, for each dose vs. placebo). A total of 50% of responders to MTNX defecated in the first 30 minutes after its administration. The use of MTNX has also been evaluated in two multi-centered, double-blind, placebo-controlled, phase III studies (33), which included 1048 patients in the recovery of postoperative ileus after intestinal resection, without finding differences between the MTNX and placebo group in a shortening in time between surgery and the first bowel movement, no reduction in hospital stay. None of the previous studies showed changes in analgesia and the side effects were mild, the most frequent being: abdominal pain, diarrhea and nausea, and vomiting. A recent meta-analysis (34) which analyzed 6 randomized clinical trials MTNX vs. placebo and that include 1,239 patients, 48.3% of them received MTNX showing a significant increase (p <0.0001) in spontaneous bowel movement compared to placebo for both 15 mg/kg and 30 mg/kg per subcutaneous route and every other day, as well as it showed safety and no effect on analgesia. The efficacy and safety of MTNX was evaluated in an open trial (35) conducted for 10 weeks in which 8 or 12 mg of MTNX were administered every other day for a weight of between 38 and <62 kg and ≥ 62 kg, respectively. A total of 149 patients with an oral morphine equivalent mean dose of 157 mg/d were included; 38.3% with 8 mg MTNX and 61.7% treated with 12 mg MTNX, showing an average weekly bowel movement of 2.2 and 3.1, respectively. The main side effects found were similar to those observed in other studies: abdominal pain (26.8%), diarrhea (16.1%) and nausea (14.1%).
Alvimopan
Alvimopan (ALVP) (trans-3,4-dimethyl.4-(3-hydroxyphenyl) piperidine) is a competitive antagonist of the mu opioid receptor, with no significant activity on the delta and kappa opioid receptors, and low affinity for non-opioid receptors (adrenergic, dopaminergic, serotonergic and peptidergic) slowly decoupling from most ligands; additionally, its affinity with mu opioid receptors is larger than MTNX and naloxone (36). Its passage through the blood-brain barrier is limited due to its large molecular size (molecular weight 424.53 g/mol), shape and high polarity (37). It is currently approved for the treatment of postoperative ileus after partial resection of the large intestine or small intestine with primary anastomosis, in those patients with postoperative opioid analgesia (38).
Pharmacokinetics
ALVP has a bioavailability of ~6% (38); in nonsurgical patients the food decreases its bioavailability by 54%, whereas in surgical patients it decreases 80% (39). ALVP reaches its maximum concentration (Cmax) after 2 hours of the administration of an oral dose. With its administration 2 times per day for 5 days, an average Cmax of 10.98 ng/ml is reached. Its binding to plasma proteins (70-80%) is independent of its concentration (38). ALVP is metabolized by the intestinal flora and its active metabolite (ADL 08-0011) is absorbed systemically without being able to demonstrate clinical relevance regarding the action of ALVP and without clinically significant contribution to the effect of the drug (38). The elimination of ALVP is mainly through the bile duct (~65%) and it has a 35% renal excretion. The elimination half-life of ALVP is 10 to 17 hours, whereas the elimination half-life of the intestinal metabolite is 10 to 18 hours. The pharmacokinetics of ALVP do not vary with weight, sex, body mass index, inflammatory bowel disease, ulcerative colitis, renal function, antibiotic therapy, antacids or P-glycoprotein inhibitors (amiodarone, diltiazem, cyclosporine, itraconazole, quinine, quinidine, spironolactone or verapamil) (39). In contrast, the pharmacokinetics of its metabolite ADL 08-0011 is affected by the use of oral antibiotics, race, inflammatory bowel disease, antacids, although with little clinical relevance (39).
Clinical trials
In a randomized, double-blind, crossover, placebo-controlled phase II study conducted in 14 healthy volunteers in whom morphine (0.05 mg/kg/EV), ALVP (4 mg) and placebo were administered; GI passage was extended by morphine from 69 to 103 minutes, which was prevented by ALVP (p = 0.005) (37). In the same study (37), but with a parallel design, the effect of ALVP on analgesia (0.15 mg/kg/IV morphine) was evaluated in 45 patients undergoing third molar exodontia, finding that analgesia and pupillary contraction were not affected by ALVP (p <0.002). In another phase II study conducted in 79 patients undergoing partial colectomy, hysterectomy or bowel resection, the efficacy of ALVP in accelerating GI recovery was assessed; a reduction in time for the first flatulence was found in the ALVP arm 70 vs. 49 h (p = 0.01) and first bowel movement 91 vs. 68 h (p = 0.03). Likewise, the average time for discharge decreased from 91 h to 68 h (p = 0.03) (40).
Post-operative ileus
Wolf et al. (41) conducted a multicenter, randomized, double-blind, placebo-controlled phase III study and an intention-to-treat analysis of 469 patients undergoing bowel resection or total hysterectomy who were randomized for 6 mg ALVP, 12 mg ALVP or placebo administered up to two hours before surgery and post-intervention every 12 hours until discharge, and up to 7 days. The mean GI recovery period was accelerated by 15 h for 6 mg ALVP (p <0.05) and 22 h for 12 mg ALVP (p <0.001) compared to placebo, and the mean discharge time compared to placebo was reduced (146 h) for the dose of 6 mg ALVP (133 h) and 12 mg ALVP (126 h) (p = 0.003). The most frequent side effects were nausea (54.5%), vomiting (19.9%) and hypotension (13.6%). Consistent results were obtained in a phase III study, which evaluated 451 patients, with the exception that the recovery of GI function was not significant for 12 mg ALVP (p = 0.059) (42), whereas another study of similar design recruiting 615 patients showed efficacy for 12 mg ALVP, but not for 6 mg ALVP (43). In contrast, the study conducted by Büchler et al. (44) (911 patients) showed no efficacy for either of both doses. An analysis of three phase III studies (41-43) showed a decrease in side effects related to postoperative ileus (use of SNG, anastomosis leaks, nausea and vomiting, and abdominal distension) in the 12 mg ALVP arm compared to placebo (45). Herzog et al. (46), in a phase III trial, of the same design as those mentioned above, conducted in 519 patients in which total hysterectomy was performed showed benefit of 12 mg ALVP compared to placebo in GI recovery.
Opioid-induced constipation
Webster et al. (47) investigated the efficacy and safety of ALVP in 522 patients with non-cancer pain and OIC in a 6-week, double-blind phase IIb study, with post-randomization in four branches ALVP 0.5 mg/12 h, 1 mg/24 h, 1 mg/12 h and placebo. Inclusion criteria were: <3 spontaneous weekly stools and at least one of the following symptoms in ≥ 25% of bowel movements: feeling of incomplete evacuation, straining or hard or caprine feces. The primary objective was the change in the number of weekly spontaneous bowel movement during the first three weeks. The three dosages of ALVP significantly increased the mean number of spontaneous bowel movement compared to placebo (p ≤ 0.01). The percentage of patients with ≥ 3 spontaneous weekly stools for the 6-week follow-up were: 14% for placebo, 38.8% for 0.5 mg/12 h ALVP, 39.5% for 1mg/24 h ALVP and 42.1% for 1 mg/12 h ALVP. The most frequent adverse effects were abdominal pain (17-28%), diarrhea (7-14%) and nausea (7-10%). Three patients presented cardiac events in the 1 mg/24h ALVP arm, which although not related to ALVP, it led the FDA to limit ALVP to hospital use and for short periods (15 doses) and it is contraindicated in patients treated with opioids for more than one week (48). Similar efficacy results were obtained in another phase III study that evaluated the response in 518 randomized patients in three arms: 0.5 mg/24 h ALVP, 0.5 mg/12 h ALVP or placebo for 12 weeks, although the ALVP development program for OIC had already been suspended. Irving et al. (49) in a phase III, placebo-controlled study conducted in 485 patients with non-cancer pain and OIC, patients were randomized to receive 0.5 mg/24 h ALVP, 0.5 mg/12 h ALVP or placebo for 12 weeks. The main objective was the proportion of responders (≥ 3 spontaneous weekly stools and an average increase ≥ 1 of spontaneous weekly stools compared to the beginning of the study). The proportion of responders was not significant compared to placebo for any of the dosages tested (0.5 mg/24 h ALVP; p = 0.259 and 0.5 mg/12 h ALVP; p = 0.214). The most frequent adverse effects were those observed in previous studies.
Naloxegol
Naloxegol (NLXG) is a polyeglycol (PEGylated) conjugate of naloxol, which is also a derivative of naloxone. NLXG has a structure based on a morphinan ring, which shares with methylnaltrexone, but different from alvimopan (38), with a molecular weight of 637.7 g/mol. PEGication decreases the first hepatic passage of NLXG and limits penetration into the central nervous system by reducing the passive permeability of the blood-brain barrier. Naloxegol is currently approved by the European Medicines Agency (EMEA) and the US Food and Drug Administration (FDA) for the treatment of opioid-induced constipation (11).
Pharmacodynamics
NLXG is a neutral opioid receptor antagonist, that is, it lacks intrinsic activity and, therefore, it does not respond in the absence of the agonist, in this case an opioid. Compared to naloxone, NLXG has shown 33 times less potency in antagonizing morphine in the GI tract and it is 49 times less potency in antagonizing morphine-produced analgesia (11). Likewise, NLXG has shown a brain reuptake 15 times lower than naloxone (11). Compared to methylnaltrexone, NLXG as an opioid antagonist has shown 3 times greater affinity for human mu opioid receptors and 9.4 times for human delta opioid receptors than methylnaltrexone; no significant differences were found for murine Kappa receptors (11).
Pharmacokinetics
In healthy volunteers 25 mg NLXG (50) is quickly absorbed orally (~ 15’) reaching a plasma Cmax of 51 ng/ml in a median time of 1.74 hours (range 0.25-3.02), with a second peak ~ 4 hours, attributable to enterohepatic recirculation. Its bioavailability is ~ 62 %, increasing about 45% when administered with food, which makes it advisable to administer on an empty stomach (51). Furthermore, when the tablets are crushed and dissolved for oral or Nasogastric tube administration, the bioavailability of NLXG is not affected (52). Its binding to plasma proteins is 4.2%. The elimination half-life is 7.88 hours. It is rapidly metabolized through cytochrome P450 (CYP3A4/5) via demethylation, oxidation, dealkylation and shortening of the polyethylene glycol chain. This has been shown to be relevant, since drugs that inhibit CYP3A4/5 substantially increase plasma levels of NLXG (53), and those inducing enzymatic action, such as rifampicin, decrease these levels markedly (53). Concomitant administration of a weak CYP3A4 inhibitor and potent P-glycoprotein, such as quinidine, has not shown a significant increase in crossing the blood-brain barrier by NLXG (54,55). The administration of morphine and NLXG together did not alter the pharmacokinetic properties of both drugs. (54,55). Six metabolites have been determined, being carboxy-methyl-PEG4-naloxol the most abundant (9.5%). Elimination is essentially fecal, eliminating unchanged 16% in feces and 5.9% in urine (50).
The administration of NLXG 25 mg in patients with moderate renal failure (GFR 30-59 ml/min/1.73 m2) and severe (GFR <30 ml/min/1.73 m2) has a low impact on the pharmacokinetics of the drug, but given that renal failure can lead to a decrease in liver and intestinal metabolism and an increase in biliary excretion with an increase in the bioavailability of NLXG, it is recommended to reduce its dose by 50% (56). Regarding patients with mild (Child-Pugh A) or moderate (Child-Pugh B) liver involvement, the pharmacokinetics of NLXG are not significantly affected, so no dose adjustment is required (57).
Regarding age, although it does not appear to have a significant effect on the pharmacokinetics of NLXG, elderly patients (median age 72 years) exposure to NLXG in steady state has shown to be increased by 54%, so caution regarding repeated administration is recommended (58). The pharmacokinetics of NLXG is not affected by sex, race, body weight or body mass index (58).
Clinical trials
A multicenter, randomized, double-blind, placebo-controlled, dose escalation (5 mg, 25 mg and 50 mg NLXG) phase IIb trial (59) included 207 adult patients treated with opioid for both cancer and non-cancer pain, and with a stable oral morphine equivalent daily dose between 30 and 1,000 mg in the two weeks prior to screening, presenting symptoms of opioid-induced constipation (<3 weekly spontaneous bowel movements and at least one of the following symptoms: hard stools, straining, feeling of incomplete evacuation, feeling of anorectal obstruction) and who agreed to discontinue the laxative treatment during the trial. The patients were stratified based on the oral morphine equivalent daily dose: low (30-100 mg) and high (> 100-1,000 mg). The main objective of efficacy was the average in the change of weekly spontaneous bowel movement between the baseline (before inclusion) and the two weeks of treatment. The patients remained in the randomization phase for 4 weeks. In the event that there were no bowel movements within 72 hours, rescue bisacodyl was allowed. Both patients with doses of 25 mg (p = 0.002) and 50 mg (p = 0.0001) obtained a significant change in the main objective, compared with placebo. Also, during the 4 weeks of randomization, the median change compared to baseline remained significant for 25 and 50 mg, p = 0.0022 and p <0.0001, respectively. The results were equally consistent for both opioid doses. The median time for the first spontaneous deposition was 6.6 h for 25 mg and 2.9 h for 50 mg. Regarding safety, it should be noted that the incidence of adverse effects in patients with 50 mg NLXG was higher than with placebo, while in the 25 mg group the incidence of adverse effects was similar to placebo. The most frequent side effects were abdominal pain, diarrhea and nausea. During the study, no significant changes in analgesic requirements or symptoms due to opioid deprivation were found. For the dose of 5 mg NLXG, no benefit or adverse effects other than placebo were found. Ultimately, taking into account the efficacy and safety, the administration of 25 mg NLXG once a day was considered the appropriate dosage and dosing regimen for future phase III trials.
Chey et al. (60) published two parallel, multicenter, randomized, double-blind, placebo-controlled phase III trials to determine the efficacy and safety of NLXG. A total of 1,352 outpatient adult patients (18 to 84 years) were included, these patients were under opioid treatment for non-cancer pain with a stable oral morphine equivalent daily dose between 30 and 1,000 mg and presented symptoms of opioid-induced constipation (<3 weekly spontaneous bowel movements or at least one of the following symptoms in 25% of bowel movements in the 4 weeks prior to screening: hard stools, straining, feeling of incomplete evacuation, feeling of anorectal obstruction). The patients were randomized 1:1:1 in three arms: 12.5 mg/d NLXG, 25 mg/d NLXG, and placebo, 1 daily dose for 12 weeks per intention-to-treat. The use of laxatives was not allowed during the study, allowing 10-15 mg bisacodyl and, if necessary, an enema when there were no stools ≥ 72 h. The main objective was the response rate during the 12 weeks of treatment; considering as response the existence of ≥ 3 spontaneous bowel movement per week and an increase ≥ 1 bowel movement compared to the depositions at the time of inclusion, in ≥ 9 of the 12 weeks of the study and ≥ 3 weeks of the last 4 weeks of the treatment period.
Regarding the patients, more than 50% were under opioid treatment for low back pain, with an average treatment of more than 3 years. A total of 71% of patients used laxatives in the 2 weeks prior to inclusion; 67% approximately used one type of laxative, and 30% approximately used two types of laxatives, usually softeners and stimulants.
Compared to placebo, in one of the studies the objective was achieved for both 12.5 mg naloxegol (p = 0.02) and 25 mg naloxegol (p = 0.001), with statistically significant response rates of 11.5% and 15%, respectively; whereas in the other study the objective was only achieved with the dose of 25 mg (p = 0.02) with a response rate of 10.3% (95% CI: 1.7 to 18.9). Additionally, a considerable improvement in straining, stool consistency and stool frequency was found, especially with 25 mg NLXG compared to placebo. Likewise, the proportion of patients who required rescue laxatives during the study was 72%, 63.4% and 54.7% for placebo, 12.5 mg NLXG and 25 mg NLXG, respectively. The median time to appear the first spontaneous bowel movement was 5.9 h and 12 h in the 25 mg groups. Regarding safety, patients were taking NLXG ~ 90% of study days. Side effects were mild and occurred more frequently in the 25 mg groups; especially abdominal pain (12.6-19%), diarrhea (9.1-9.3%), nausea (7.5-8.6%), flatulence (5.6-6%) and vomiting (2.8 -6%). In both studies, 10.3% presented adverse effects that led to discontinuation of the drug. The average dose of opioid remained stable throughout the study period, only 5 patients presented with symptoms of opioid deprivation.
NLXG was intended to be studied in cancer patients (61) with OIC but recruitment after 10 months was 4% of the planned population (14 out of 336). The researchers cited different causes for low recruitment such as the prioritization of other research and that the protocol was too restrictive or inconsistent with the usual management of cancer patients, such as the stability of the opioid dose for 4 weeks.
The long-term safety and tolerance of NLXG was studied in a 52-week parallel, multicenter, open, randomized phase III trial (62). A total of 844 patients were randomized, 84 from the previous phase III trials, and 760 as new patients. The inclusion and exclusion criteria were the same as those previously described for phase III trials. The patients were randomized 2:1 (25 mg/day NLXG: usual treatment with laxatives). The demographic characteristics and the causes of pain were similar between both groups, 87.4% had musculoskeletal origin, especially back pain (52.9%). Regarding the type of opioid and proportion of patients who used them in both groups, there were no significant differences, the most commonly used analgesics were: hydrocodone + acetaminophen (paracetamol) (32.3%), morphine (27.9%), oxycodone (25%), oxycodone + acetaminophen (18.9%) and tramadol (13.6%). The average oral morphine equivalent daily doses were 151.5 mg and 136.7 mg for the NLXG and laxative groups, respectively. The previous mean time of opioid treatment was ~ 4 years in both groups. Additionally, 98.3% took other medications: benzodiazepines (41.4%), antidepressants (31.1%) and statins (30.5%), among the most frequent. Patients were taking NLXG for 73.6% of the days of study and treatment with laxatives for 81.3% of the days. A total of 73% of patients with laxatives did not change them throughout the study. Most of the side effects were mild or moderate, although more frequent in the NLXG group compared to the laxative group: abdominal pain (17.8% vs. 3.3 %), diarrhea (12.9 % vs. 5.9%) and sickness (9.4% vs. 4.1%), which was interpreted as expected according to the mechanism of action of NLXG. Regarding other adverse effects of special interest such as cardiovascular events (2 patients), hypotension (10), high blood pressure (33), GI (6), bowel perforation (0), opioid deprivation (3), all similarly distributed in both study arms and none attributable to the drugs used. Both pain scores and opioid doses remained stable throughout the study. Finally, 327 patients in the NLXG arm completed the study and 189 in the treatment with laxatives; whereas 207 (36.7%) and 81 (28.8%) discontinued treatment, respectively. The results show that 25 mg/day NLXG is safe and well tolerated without interfering with opioid analgesia.
DISCUSSION
The PAMORA described above have shown efficacy in the treatment of OIC compared to placebo and in different patient populations, and in the case of NLXG compared to laxatives (62). MTNX was studied and approved for patients with cancer pain or other diseases in advanced stages (30,31-34), whereas ALVP (41,42-46) and NLXG (60,62) have been for non-cancer patients, although its use in cancer patients is accepted in the NLXG data sheet. Recently, naldemedine has been approved by the Spanish Agency of Medicines and Medical Devices (AEMPS, Agencia Española de Medicamentos y Productos Sanitarios in Spanish) as a new and promising PAMORA for the OIC indication (63), although it has not yet been commercialized in Spain. In relation to the effectiveness of the different PAMORA, each one has shown to be significantly more effective compared to placebo, but no face-to-face studies between different PAMORA are available to date. An indirect comparison is also not possible, since the response parameters for the three drugs are different: for MTNX the spontaneous bowel movement is within the first 4 h after administration, whereas for NLXG it is the existence of ≥ 3 spontaneous bowel movement per week, which is more consistent with the OIC criteria of ROME IV (9,64). In the case of ALVP, the primary objective is the recovery of the GI function after surgery, and studies for its development as an oral treatment of OIC, with the same response criteria to those of the NLXG studies, were canceled because of the cardiac risk (48). Regarding the side effects, the safety profile is similar for the most prevalent symptoms: abdominal pain, diarrhea, nausea/vomiting, not so much for the cardiac risk with ALVP, already mentioned. Additionally, the potential interactions of NLXG with drugs that share the CP450 pathway must be taken into account (53), as well as the use of NLXG (56) and MTNX (24,25) in renal failure (Table III).
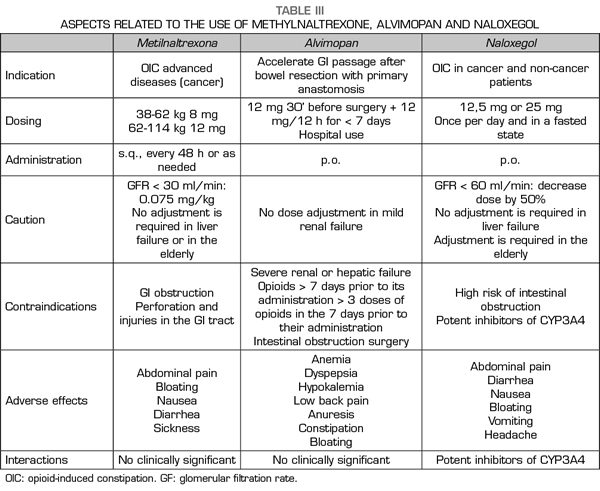
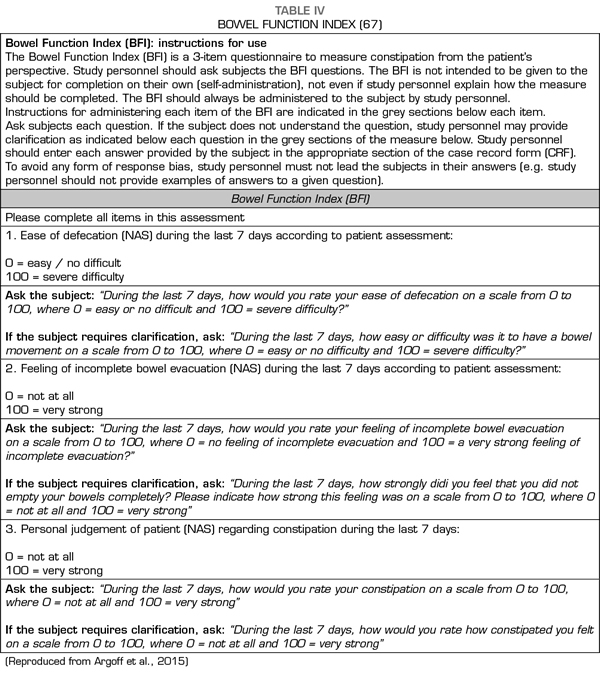
Only a proportion of patients with OIC did not respond to the administration of PAMORA in the different studies and required rescue laxatives. This suggests that the pathophysiological mechanisms of constipation, even in the presence of opioids, are more complex and that other causes (such as drugs with anticholinergic action (13) or intestinal dysfunction related to the use of chemotherapeutics [65] should be considered. Therefore, although PAMORA constitutes an etiopathogenic OIC approach, this does not obviate the use of laxatives and questions whether laxatives and PAMORA should be used concomitantly, or if PAMORA should be used as rescue in patients who do not respond to laxatives (66) remain to be evidence-based answered. The use of laxatives has been proposed as a first line in constipation in patients with advanced diseases (6), restricting the use of PAMORA for refractory cases; furthermore, the use of PAMORA has been recommended for patients with a Bowel Functional Index (BFI) ≥ 30 (67). The BFI has been validated for the diagnosis of OIC (68,69) (Table IV).
Apart from the OIC, it has been hypothesized about the use of PAMORA in pruritus secondary to the use of opioids (70), or in the case of cholestasis (71); in this sense, a relieve of itching has been reported in cholestasis in patients with advanced cancer treated with MTNX (72), but no relieve was found in the itching associated with the administration of spinal opioids (73,74). This may seem marginal because of its frequency, but it deserves to be studied, especially pruritus associated with cholestasis in patients with advanced cancer.
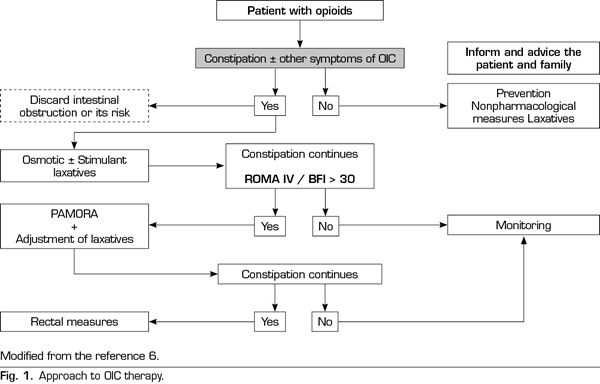
Based on the available information, it seems appropriate to consider that an approach to the patient under opioid treatment (Figure 1) should continue to consider the laxative treatment as the first option, taking into account that the combination of osmotic and stimulant laxatives is usually more effective than the laxative only (75). If despite the laxative treatment the patient meets the criteria of OIC ROME IV and the BMI is> 30, the use of PAMORA should be the next logical step. In any case, the verification of a limited effect of laxatives should not delay the indication of a PAMORA, although it may be more realistic and consistent with the clinic to start PAMORA and laxatives concomitantly reducing the doses of the latter and adjusting the dose of laxatives according to the patient’s response. All of this should always be based on adequate information of realistic expectations, for both the patient and the family.
All PAMORA described above have been approved by the European Medicines Agency (EMEA) and the US Food and Drug Administration (FDA) in the treatment of OIC; in the particular case of ALVP only to accelerate the GI recovery after intestinal resection with primary anastomosis. In Spain, MTNX was marketed from July 2009 to September 2012, and ALVP is not available. At present in Spain only NLXG is available in tablets of 12.5 and 25 mg.
CONCLUSIONS
A better diagnosis of OIC in the clinical context seems imperative, in which the use of validated instruments such as BFI and criteria such as ROMA IV can certainly be of great help. All available PAMORA have shown efficacy in the OIC, but it remains open what should be their place in the therapeutic scheme of the treatment of the OIC, their use in laxative-refractory OIC or their concomitant use. Additionally, the potential benefit of PAMORA in refractory pruritus due to cholestasis should be assessed. All these are key aspects to be addressed in further studies.
CONFLICTS OF INTEREST
CCS, ALB and JPS have participated in informative activities promoted by Kyowa Kirin Farmaceutica España.
REFERENCES