DOI: 10.20986/resed.2020.3797/2020
ORIGINAL
Incidence of post-mastectomy pain syndrome. Retrospective analysis
Incidencia del síndrome posmastectomía. Análisis retrospectivo
M. Mayo Moldes1
T. Fernández Rodríguez1
G. Illodo Miramontes1
A. Carregal Raño1
M. J. Goberna Iglesias1
1Unidad de Dolor Crónico, Hospital do Meixoeiro. Vigo, Pontevedra, Spain
ABSTRACT
Introduction: The posmastectomy pain syndrome (PMPS) is included in the new ICD-11 (International Classification of Diseases 11th Revision) classification under the postsurgical pain syndrome subset. Waltho and Rockwell have recently consensuated what they call post breast surgery pain syndrome (PBSPS) and conclude that a complete definition of PBSPS is pain that occurs after any breast surgery; is of at least moderate severity; possesses neuropathic qualities; is located in the ipsilateral breast/chest wall, axilla, and/or arm; lasts at least 6 months; occurs at least 50 % of the time; and may be exacerbated by movements of the shoulder girdle. The published incidence of PMPS varies between 11-57 % according to different sources. The incidence lowers to 5-10 % when the pain is judged severe. Up to the 65 % of the patients present neuropathic characteristics. The aim of this article is to primary to determine the frequency and intensity of the incidence of, and seconddly to elucidate possible risk factors to influence its appearance.
Material and method: A retrospective analysis, of all partial or total mastectomies with or without axillary nodal resection, performed between January the 1st 2017 December 31st 2017, was carried out.
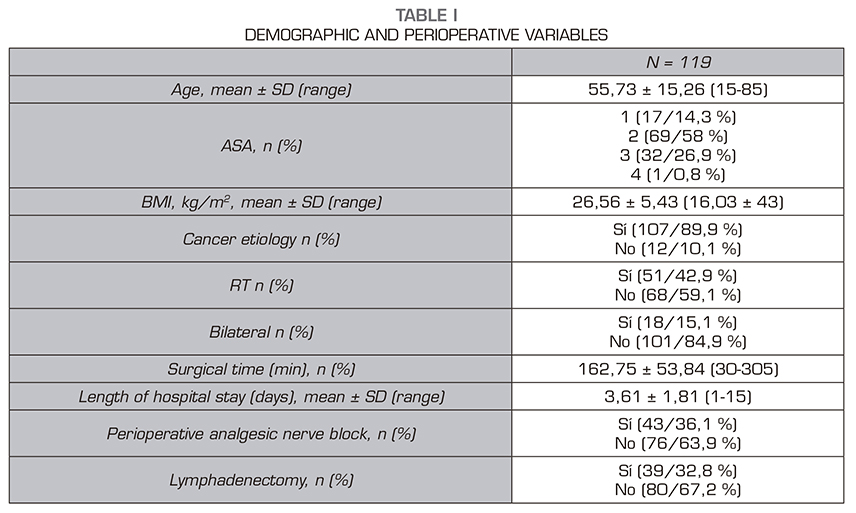
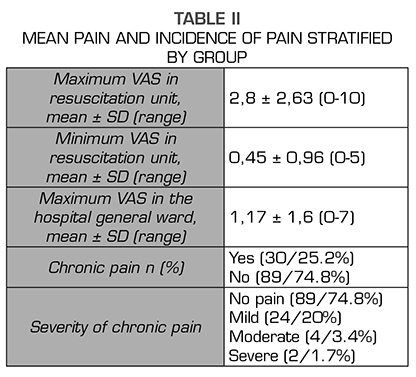
Results: A total number 119 patients were studied, 30 of which (25,2 %) declared to feel pain. The intensity was low in 24 patients, moderate in 4 and severe in 2. No relationship was found between the appearance of chronic pain and any of the variables studied.
Discussion: We judge necessary to perform new prospective studies which include a consensuated definition of PMPS to clearly elucidate possible risk factors that can contribute either to the incidence, intensity or impact in quality of life of our patients.
Key words: Postmastectomy pain syndrome, postsurgical chronic pain, neuropathic pain, oncological pain.
RESUMEN
Introducción: El síndrome de dolor posmastectomía (SDPM) aparece recogido en la nueva clasificación ICD-11 (International Classification of Diseases 11th Revision) como un subgrupo de dolor crónico posquirúrgico. Waltho y Rockwell han consensuado recientemente en un artículo de revisión lo que ellos denominan síndrome de dolor poscirugía mamaria (estos criterios han sido publicados en nuestra lengua en una carta al Director en la RESED), que describen esta situación como un dolor que se produce después de cualquier cirugía de mama; es de, al menos, intensidad moderada, presenta características neuropáticas, se localiza en la mama, pared torácica, axila o brazo ipsilateral, tiene una duración mínima de 6 meses, ocurre al menos el 50 % del tiempo y puede ser exacerbado por los movimientos de la cintura escapular. Es probablemente secundario a una lesión del nervio intercostobraquial o intercostales durante la disección a nivel axilar, lo que explica las características neuropáticas de este dolor. La incidencia documentada de SDPM varía según las publicaciones entre un 11 y un 57 %, bajando a un 5-10 % en caso de que sea severo, destacando que hasta el 65 % de los casos presentan características neuropáticas.
El objetivo primario de este trabajo es determinar la frecuencia y la intensidad de la incidencia del SDPM y el secundario identificar posibles factores de riesgo para su desarrollo.
Materia y métodos: Se realizó un análisis retrospectivo de todas las mastectomías parciales o totales con o sin vaciamiento ganglionar realizadas entre el 1 de enero de 2017 y el 31 de diciembre 2017.
Resultados: De los 119 pacientes analizados, 30 pacientes refirieron dolor (25,2 %). La intensidad fue leve en 24 pacientes, moderado en 4 y severo en 2. No hubo relación entre el riesgo de dolor crónico y cualquiera de las variables analizadas.
Discusión: En nuestra opinión, parece necesario realizar nuevos estudios prospectivos que incluyan una definición consensuada del SDPM para evaluar los posibles factores de riesgo que afecten tanto en incidencia, intensidad e impacto en la calidad de vida de nuestros pacientes.
Palabras clave: Dolor posmastectomía, dolor crónico posquirúrgico, dolor neuropático, dolor oncológico
Received: 24-02-2020
Accepted: 09-05-2020
Correspondence: Mónica Mayo Moldes
mayomonica@hotmail.com
INTRODUCTION
At the end of the last century, one of the first articles on pain associated with surgery and trauma was published in the United Kingdom (1), and at that time Macrae & Davies defined the chronic postsurgical pain (CPSP) for the first time (2). In the first decade of this century, Macrae published a review on the subject where the author reflects on the definition and problems in research (3), and at the end of the last decade a new study was published reviewing the definition and situation of the problem (4). The studies mentioned above and others raised awareness among the scientific community on the importance of the problem and they promoted a change in the assessment of the importance that chronic postoperative pain involves in both the physical and emotional suffering of patients and its economic burden. In a symbolic way, we can understand that the efforts promoted by the IASP with the proclamation of 2017 as “Global Year Against Pain After Surgery” (5) and its recent incorporation of the term chronic postsurgical pain or post-traumatic pain in the ICD-11 (6), as recognition of the effort of all these researchers to promote a change of mentality in the perception of this problem in health, government and patient organizations.
The review of Werner & Kongsgaard (4) defines PSCP as pain that develops or increases after a surgical procedure, the duration of pain should be of at least 3-6 months, and it should affect the patient’s quality of life (Health-related quality of life [HR-QOL]); pain is a continuation of acute postoperative pain or develops after an asymptomatic period. The pain is located to the surgical field, projected to the innervation territory of a nerve situated in the surgical field, or referred to a dermatome; and, finally, other causes of the pain should be excluded (4).
Post-mastectomy pain syndrome (PMPS) has recently been defined by Waltho and Rockwell (7) (these criteria have been published in Spanish in a letter to the Director in the RESED) (8), they describe this situation as: a pain that occurs after any breast surgery; is of at least moderate severity, possesses neuropathic qualities, is located in the ipsilateral breast/chest wall, axilla and/or arm, lasts at least 6 months, occurs at least 50 % of the time, and may be exacerbated by movements of the shoulder girdle. It is probably secondary to an injury in the intercostobrachial or intercostal nerve during axillary dissection, which explains the neuropathic characteristics of this pain (9,10,11). The PMPS appears in the new ICD-11 classification as a subset of CPSP. 6
The documented incidence of PMPS varies according to publications between 11-57 %, falling to 5-10 % if severe; up to 65 % of cases have neuropathic characteristics (12).
The aim of this study was to determine the frequency and severity of chronic pain after mastectomy and to identify potential risk factors for its development.
PATIENTS AND METHODS
A retrospective analysis of all partial or total mastectomies with or without axillary lymph node dissection was performed at the Meixoeiro Hospital in Vigo between January 1, 2017 and December 31, 2017. The study protocol was approved by the Ethics committee (No. 486) and all patients except one provided their consent to be included in the study. Male patients, reinterventions, and deceased patients were excluded from the analysis.
A telephone questionnaire was applied to all patients meeting the inclusion criteria one year after surgery to assess the presence, location, intensity, and the duration of the pain. Patients who were not located after three calls were excluded from the study.
The study variables were collected from the hospital database.
The primary variable was postsurgical chronic pain evaluated one year after the surgery; the pain existence variable and the pain intensity variable evaluated by the verbal numerical scale (VNS) were collected, ranging from 0 (no pain) to 10 (maximum pain). For the present study, three standardized pain intensities were established: Mild pain VNS ≤ 3, moderate pain 4-7, severe pain ≥ 8.
Secondary variables evaluated were: Maximum postoperative pain in the resuscitation unit, maximum pain in the hospital general ward, demographic variables: age, sex, BMI; previous physical status stratified using the anesthetic risk scale (American Society of Anesthesiologists, ASA), surgical indication (mastectomy of oncological etiology or not), whether lymphadenectomy was required, time of surgery, type of anesthesia (general or combined), and whether radiation therapy was required as adjuvant therapy.
The results are expressed as number of cases and percentage for categorical variables. Results are expressed as mean ± standard deviation for quantitative variables. The comparison of variables with postoperative chronic pain was performed with the Chi-square test. The Student t test or ANOVA test were used for quantitative variables and the Fisher Chi-square and exact tests were used for qualitative variables. A multivariate analysis with logistic regression was performed with clinically relevant results and statistically significant differences in the bivariate analysis. The Hosmer and Lemeshow goodness-of-fit test was used to validate this model. The statistical analysis was performed using the SPSS program (version 25.0). The probability of a type I error was set at 5 % ( = 0.05) with a bilateral approximation.
RESULTS
In the study period, a total of 654 surgeries were performed in the breast unit, 130 of them were mastectomies. One male patient and 8 deceased patients were excluded. A total of 121 patients were contacted, 2 of them were excluded from the analysis, one of them refused to participate and the other was not located after 3 calls, leaving 119 patients (91.5 %) for analysis.
Thirty of these 119 patients reported pain (25.2%): mild pain in 24 patients, moderate pain in 4 patients, and severe pain in 2 patients. Table 1 shows the demographic and perioperative variables. Table 2 shows the incidence of pain stratified by age groups, ASA, cause of surgery, perioperative radiation therapy, unilateral versus bilateral mastectomy, lymphadenectomy and type of anesthesia. No relationship was found between the risk of chronic pain and any of the variables analyzed.

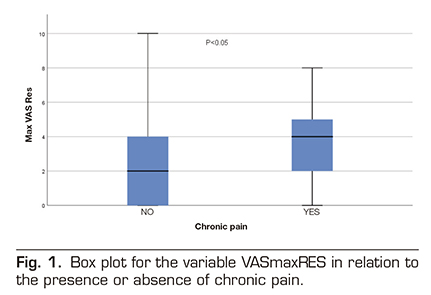
When we analyzed the other variables (surgical time, length of hospital stay, maximum pain in resuscitation, or maximum pain in hospital general ward) we found in the box plot for the variable VAS Maximum in resuscitation (VASmaxRES) in relation to the presence or absence of chronic pain that in this distribution there may be differences between both groups (those patient with chronic pain and those patients without chronic pain). For all other quantitative variables, the distribution of the box diagram was similar between both groups. A Student t test confirmed that the difference between both groups in relation to the maximum VAS in resuscitation is significant (p = 0.02) (Figure 1). We then performed a logistic regression model with all the variables. The variable VASmaxRES still showed significant differences in the model, but none of the other variables showed significant differences (Table 3).
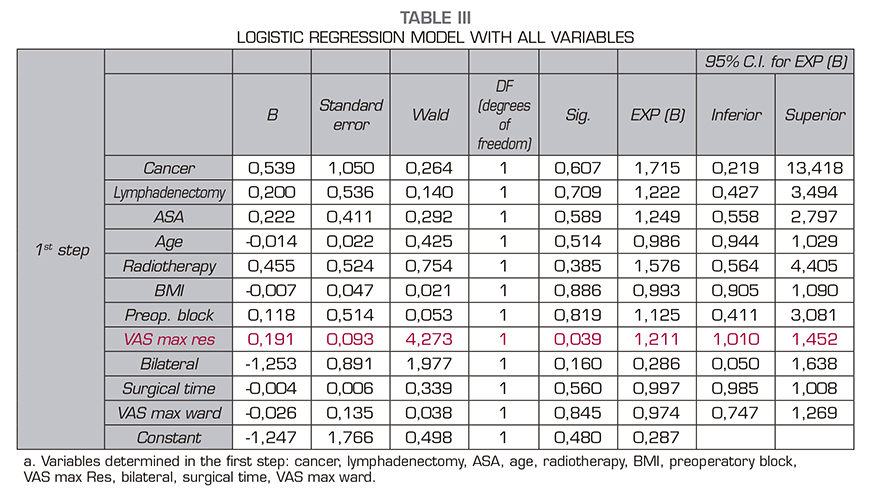
The goodness-of-fit test was significant (p < 0.05), which means that our model does not fit our observations correctly. The binary logistic regression model with a McFadden of the pseudo R-square of 0.0340 is very low for our model; this value would indicate thaat our model would only correctly explain 6% of the occurrence of the dependent variable. Therefore, this model is not valid to explain the development of chronic pain after mastectomy.
In the case of the first and main objective of the study, to calculate the incidence of PMPS in our population, we have found the proportion and its 95 % confidence interval. The estimated margin of error in our study was 8% for n = 119.
DISCUSSION
Post-mastectomy pain syndrome (PMPS) is a clinical condition that has not been well defined, although several definitions have been used in the literature. This has resulted in a methodological difference in clinical studies, so these results cannot be extrapolated between the different studies because of the lack of a standardized definition and the reference criterion is in many articles the diagnosis of the experienced clinician based on clinical practice guidelines (13).
The incidence of PMPS is described in the literature with a mean of 36.2 %, ranging between 13 and 69 % (10, 11).
In our study, 30 out of the 119 studied patients reported pain of different intensity one year after their surgery, representing 25.2% of the total.
A total of 24 out of 119 patients reported mild pain, 4 patients reported moderate pain, and 2 patients reported severe pain. Vilholm et al. (14) found that 23% had chronic pain consistent with post-mastectomy when surveyed patients with oncological mastectomy and compared them with a group of women with breast cancer who received non-surgical treatment. A total of 6% of these women reported that their pain affected “too much” or “a lot” their daily activities. In the study conducted by Vilholm et al., patients had a chronic pain incidence that was very similar to our study: 23.9% versus 25.2% in our study. In addition, 6% of these patients with PMPS had moderate/severe chronic pain compared to 5.1% in our study. Therefore, both studies have similar incidences. Consistent incidences were previously described in the cohorts studied by Carpenter et al. 27 % (15). However, we found other studies, such as the study of Perkins & Kehlet, who found an incidence of chronic pain of 50% for mastectomy (16) and Smith et al. who found an incidence of 43% (17). All these inconsistencies are explained by the different criteria for establishing the PMPS and the associated symptoms.
In the literature, several risk factors have been associated with the development of PMPS after a breast cancer surgery (18,19). In February 2014, Couceiro et al. published a study conducted in 250 patients, the authors found PMPS in 44.4 % of patients and after evaluating different variables, such as social characteristics, biological characteristics, dysmenorrhea, headache, acute postoperative pain and type of surgery, they concluded that the PMPS was associated with lumpectomy with axillary lymph node dissection, a previous history of headache, and younger than 50 years (20). Johannsen et al. conducted a nationwide study to investigate the prevalence and risk factors of PMPS in two periods after surgery (15 months and 7-9 years), including 3343 patients, 1905 of them were analyzed. A total of 32.7% of the patients reported a daily pain after 15 months, decreasing the prevalence of pain after 7-9 years to 20.4%. Factors significantly associated with pain after 15 months were young age, low cultural level, axillary surgery, more aggressive surgery, postmenopausal endocrine treatment, toxic habits (smoking more than 10 cigarettes/day), obesity (BMI between 30-35%); whereas the factors associated with pain after 7-9 years where only axillary surgery, endocrine treatment and worse physical status. This suggests that the influence of risk factors changes over time (21). Consistently, Mejdahl et al. reached the same conclusion when observed a slight decrease of 45 % (N = 1090) in pain prevalence after 2 years and a decrease of up to 37 % after 4 years (22); therefore, it is important to determine when the study is performed, because the prevalence at first increases and then tends to decrease over time.
Regarding perioperative factors, although it is not the main aim of the study, no significant differences were found in the variables studied (age, ASA, cause of surgery, perioperative radiation therapy, unilateral versus bilateral mastectomy, and lymphadenectomy) or between the anesthetic and analgesic techniques used in the perioperative period. Only the maximum score in the VAS in the postoperative resuscitation unit was significantly different between both groups. However, although no significant difference was found using the multivariate model, there is a trend showing that a greater pain in resuscitation leads to a higher risk of PMPS. Perhaps these differences would become statistically significant if the sample analyzed was larger. This is consistent with the literature, where one of the factors for the onset of PMPS is the suboptimal management of perioperative pain (23,24), either with pre-incisional locoregional techniques (25) as in perioperative. A Cochrane review on how regional anesthesia can decrease the risk of chronic pain concludes that regional blocks are effective in decreasing chronic pain after breast cancer surgery (26).
At the national level, no statistical data concerning the PMPS allowing us to evaluate its magnitude and to consider the possibility of timely, and even preventive, surgeries that would reduce the incidence of PMPS are available. Our perception is that the incidence of PMPS is similar to that of other cancer patients, inconsistently with what was published by authors, such as Perkins & Kehlet (16), who reported a percentage of 50% of mastectomy patients.
The limitations of this study are due to the data has been collected only from one center (with all the biases that this involves due to different types of surgery, anesthetic techniques…); besides it is a retrospective study, therefore, it is a no randomized study.
One of the main limitations of our study is the sample size, although the study is valid to demonstrate the incidence of PMPS. We have used the model of Peduzzi et al. to calculate the number of patients required for the second aim of the study, that is, to determine the risk factors for PMPS by using logistic regression (27). Considering that we have included 11 variables in the final multivariate model, we will have a number of events per variable (EPV) of 2.72, and therefore we would need an ideal sample of 140 patients. We assume that we used a very low number of EPV, and it was distant from the 10 or even 20 recommended in the literature. For EPV below 10, as in our case, the regression coefficients can be biased in both positive and negative directions, which clearly limits the statistical power of the logistic regression in our study.
More high-quality, prospective, multicenter studies are needed to fully elucidate the incidence of PMPS and its associated risk factors.
Despite our study was not designed to find risk factors, it is noteworthy that no significant differences have been found regarding these parameters.
Although our study was designed to find the incidence of PMPS, further prospective studies seem necessary to assess potential risk factors influencing incidence of PMPS, pain intensity, and impact of PMPS on the quality of life of our patients.
It would be necessary in future studies to use a consensus definition of the PMPS.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES